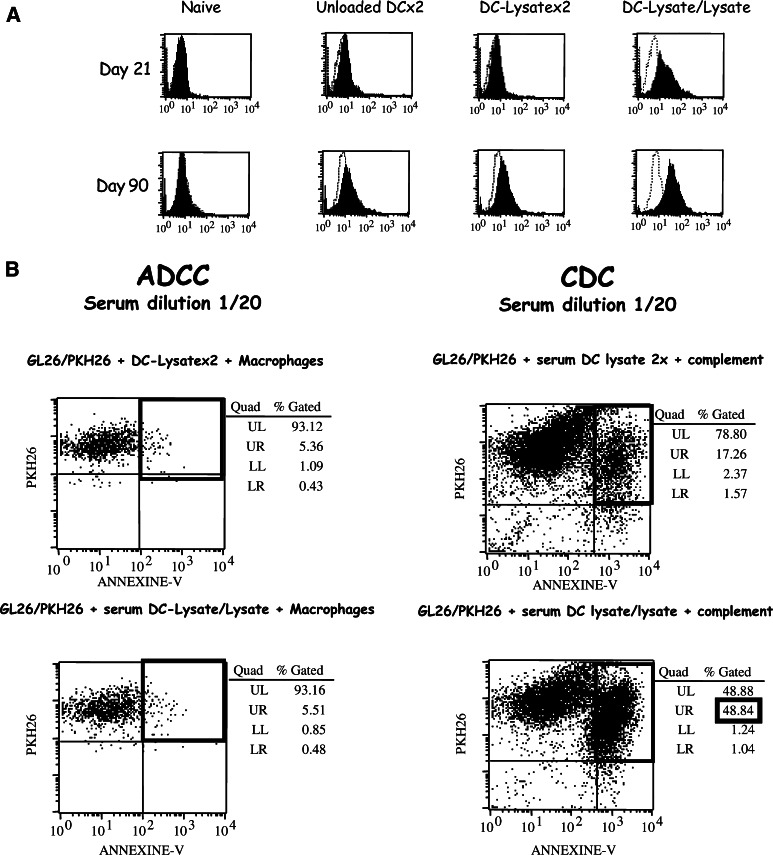
Fig. 7.
Only the DC-Lysate/Lysate protocol induces a specific humoral response mediating tumour lysis through Complement-Dependent Cytotoxicity. a Specific humoral response induced by DC-Lysate/Lysate vaccination only. Mice were vaccinated twice by unloaded DCs, DC-Lysate or with a priming by DC-Lysate followed by a boost with tumour lysate alone at days 14 and 7 before i.c. tumour implantation (Day 0). At days 21 and 90, specific mouse antibodies against GL26 were examined in the sera of mice vaccinated with unloaded-DCx2, DC-Lysatex2 or DC-Lysate/Lysate by incubation of serum dilutions (1/20) on GL26 cells. The specific binding of antibodies on GL26 cells was detected by FACS analysis after incubation with a secondary anti-mouse IgG1-FITC conjugated antibody. The in vivo vaccination with the DC-Lysate/Lysate prime/boost protocol induced a humoral response specific for GL26, detectable 21 days after the priming (the variation of mean fluorescence intensity (MFI) compared to unloaded-DC CTR was 18.1), whereas DC-Lysatex2 vaccine produced no specific antibodies (no difference of MFI compared to unloaded DC CTR). This humoral response observed in the DC-Lysate/Lysate group remained detectable 3 months post priming (variation of MFI compared to unloaded-DCx2 CTR was 42.3). b Specific anti-GL26 antibodies contribute to antitumour response through CDC and not through macrophage-mediated ADCC. Naïve mice and mice vaccinated by unloaded DCs, DC-Lysatex2, and DC-Lysate/Lysate were implanted i.c. with GL26 tumour cells (day 0). At day 90, blood samples were collected and ADCC and CDC assays were performed as described in ‘Materials and methods’ to study the mechanism of GL26 antibody toxicity. In the DC-Lysate/Lysate group, a 48.8% GL26 cell lysis (corresponding to 31.6% of specific lysis) was observed in the CDC assay compared to 17.2% of non-specific lysis in the CTR groups. No ADCC was observed