Abstract
B7-H3, a member of the B7-family molecules, plays an important role in adaptive immune responses, and was shown to either promote or inhibit T-cell responses in various experimental systems. B7-H3 was expressed in some human cancers and correlated with poor outcome of cancer patients. However, its exact role in cancer is not known. In the present study, we studied the expression of B7-H3 in the pathologic specimens of 102 patients treated for colorectal carcinoma (CRC) by immunohistochemistry. Strong B7-H3 expression was found in cancer tissues from 54.3% CRC patients, while minimal expression was found in adjacent normal colorectal tissues. Higher B7-H3 expression in tumor positively correlated with a more advanced tumor grade. In addition, consistent with a role of B7-H3 in suppressing tumor immune surveillance, the expression of B7-H3 in cancer cells negatively correlated with the intensity of tumor infiltrating T lymphocytes in both tumor nest and tumor stroma. Furthermore, we found that the level of soluble B7-H3 in sera from CRC patients was higher than healthy donors. TNF-α, an important cancer-promoting inflammatory molecule, was subsequently found to significantly increase the release of soluble B7-H3 in colon cancer cell lines. Therefore, our data suggest that both soluble and membranous B7-H3 proteins are involved in colon cancer progression and evasion of cancer immune surveillance.
Keywords: B7-H3, Tumor Infiltrating lymphocytes, Tumor necrosis factor-α, Colorectal carcinoma, Tumor immune surveillance
Introduction
Colorectal carcinoma (CRC), one of the most frequent malignancies occurring in humans, ranks as the third most common cancer and the fourth leading cause of cancer death worldwide [1]. In China, CRC has been reported to present with an increasing incidence due to the changes of diets and lifestyles [2]. CRC is a multi-pathway disease, due to the numerous pathological factors and polygene transformation that are involved in the tumor genesis and progression. Furthermore, it has been demonstrated that chronic inflammation caused by infectious or autoimmune diseases is clearly associated with increased risk of colorectal carcinoma in clinical and epidemiologic studies [3]. Within recent decades, varieties of therapeutic strategies including conventional surgery, chemotherapy, radiotherapy and immunotherapy, or combination of these therapies have been available in the treatment to CRC patients. However, the 5-year survival rate for patients diagnosed at advanced stages remains disappointing [4–6]. Therefore, considerable efforts are needed to improve current therapeutic modalities and to explore novel biologic markers to benefit the early diagnosis and targeted therapeutic interventions of colorectal carcinoma.
It is known that optimal activation of antigen-specific lymphocytes requires combination of T-cell receptor (TCR) and costimulatory signals and is negatively regulated by coinhibitory signals [7]. A cohort of B7 family ligands have been found to contribute essentially to T-cell activation and tolerance [8–13]. In fact, recent data demonstrate that some inhibitory B7 family ligands, such as B7-H1, B7-DC, B7-H3 and B7-H4, are identified to be highly expressed in a wide spectrum of human cancers, and their expression levels correlated to patient’s clinicopathological features. Moreover, increasing expression levels of inhibitory B7 molecules correlated with poor prognosis of cancer patients [14–22]. It therefore has been proposed that inhibitory B7 molecules also contribute to an immune suppressive tumor microenvironment [23]. Indeed, levels of inhibitory molecules, such as B7-H1 and B7-H4 in human solid tumor tissues inversely correlate with numbers of tumor infiltrating T lymphocytes [17, 19, 24–26].
The role of B7-H3 in adaptive immune responses still remains controversial. B7-H3 was first identified as a costimulatory molecule that engages its receptor on T cells to promote T-cell activation and secretion of IFN-γ [12, 27]. Others showed that B7-H3 plays an inhibitory role during autoimmunity [28–30]. B7-H3 is present in two isoforms, 2IgB7-H3 and 4IgB7-H3, in human beings. Besides finding cell surface expressed B7-H3, our lab reported that soluble B7-H3 could be released by monocytes, dendritic cells and activated T cells [31]. Recent studies demonstrated that B7-H3 expression on tumor cells is associated with poor outcome of patients suffering from prostate cancer or clear cell renal cell carcinoma [32–34]. However, the mechanism of how B7-H3 affects cancer progression remains unknown.
The clinical significance of B7-H3 and the regulation of its expression in human colorectal carcinoma are still elusive. In the present study, we carried out the immunohistochemistry study to characterize the B7-H3 expression in human CRC tissues and found a positive correlation with staging of CRC. In addition, we have found an inverse relationship between B7-H3 expression and the intensity of tumor infiltrating T lymphocytes, suggesting the role of B7-H3 in suppressing tumor immune surveillance. Furthermore, we have found that levels of soluble B7-H3 are elevated in sera from CRC. The level of soluble B7-H3 is enhanced by TNF-α, an important cancer-promoting inflammatory cytokine. Taken together, our study establishes an important role of B7-H3 in cancer progression and suggests an underlying suppression of cancer immune surveillance.
Materials and methods
Patients
Cancer tissues from 102 patients who underwent surgery for CRC from May 2004 to December 2007 in the Department of Gastrointestinal Surgery, the Third Affiliated Hospital, Suzhou University, Jiangsu Changzhou, China, were used in the present study. None of the patients received pre-operative chemotherapy or radiotherapy. The paraffin blocks of tumor tissues were obtained from the archival collections from the Department of Pathology, and all 102 specimens were identified as CRC under hematoxylin and eosin (H&E) staining. The patients’ pathological reports were reviewed, and their clinical parameters are shown in Table 1. In addition, 23 cases of adjacent normal tissues from autologous non-malignant portion of colon or rectum were resected from surgery to study the B7-H3 expression. This study was approved by the Ethic Committee of the hospital.
Table 1.
Correlation between clinical parameters, B7-H3 expression and T cells infiltration
| Clinical parameters | Cases | B7-H3 expression | T cells infiltration in tumor stroma | T cells infiltration in tumor nest | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Low | High | P valuea | Low | High | P value | Low | High | P value | ||
| Gender | ||||||||||
| Male | 66 | 35 (53.0)b | 31 (47.0) | 0.1018 | 41 (62.1) | 25 (37.9) | 0.1873 | 28 (42.4) | 38 (57.6) | 0.0383 |
| Female | 36 | 13 (36.1) | 23 (63.9) | 27 (75.0) | 9 (25.0) | 23 (63.9) | 13 (36.1) | |||
| Age (years) | ||||||||||
| ≤60 | 47 | 20 (42.6) | 27 (57.4) | 0.3944 | 33 (70.2) | 14 (29.8) | 0.4825 | 23 (48.9) | 24 (51.1) | 0.8425 |
| >60 | 55 | 28 (50.9) | 27 (49.1) | 35 (63.6) | 20 (36.4) | 28 (50.9) | 27 (49.1) | |||
| Tumor location | ||||||||||
| Colon | 60 | 26 (43.3) | 34 (56.7) | 0.3676 | 41 (68.3) | 19 (31.7) | 0.6695 | 32 (53.3) | 28 (46.7) | 0.4210 |
| Rectum | 42 | 22 (52.4) | 20 (47.6) | 27 (64.3) | 15 (35.7) | 19 (45.2) | 23 (54.8) | |||
| Tumor size (cm) | ||||||||||
| ≤4 | 48 | 25 (52.1) | 23 (47.9) | 0.3378 | 30 (62.5) | 18 (37.5) | 0.4000 | 24 (50.0) | 24 (50.0) | 1.0000 |
| >4 | 54 | 23 (42.6) | 31 (57.4) | 38 (70.4) | 16 (29.6) | 27 (50.0) | 27 (50.0) | |||
| Tumor (T) statusc | ||||||||||
| pT1 | 6 | 4 (66.7) | 2 (33.3) | 0.0227 | 2 (33.3) | 4 (66.7) | 0.0410 | 3 (50.0) | 3 (50.0) | 0.6797 |
| pT2 | 17 | 13 (76.5) | 4 (23.5) | 9 (52.9) | 8 (47.1) | 8 (47.1) | 9 (52.9) | |||
| pT3 | 65 | 24 (36.9) | 41 (63.1) | 47 (72.3) | 18 (27.7) | 32 (49.2) | 33 (50.8) | |||
| pT4 | 14 | 7 (50.0) | 7 (50.0) | 10 (71.4) | 4 (28.6) | 8 (57.1) | 6 (42.9) | |||
| Nodal (N) metastasis | ||||||||||
| Without | 54 | 25 (46.3) | 29 (53.7) | 0.8700 | 35 (64.8) | 19 (35.2) | 0.6739 | 29 (53.7) | 25 (46.3) | 0.4275 |
| With | 48 | 23 (47.9) | 25 (52.1) | 33 (68.8) | 15 (31.2) | 22 (45.8) | 26 (54.2) | |||
| Distant metastasis (M)d | ||||||||||
| Without | 88 | 41 (46.6) | 47 (53.4) | 0.8124 | 58 (65.9) | 30 (34.1) | 0.6841 | 43 (48.9) | 45 (51.1) | 0.5650 |
| With | 14 | 7 (50.0) | 7 (50.0) | 10 (71.4) | 4 (28.6) | 8 (57.1) | 6 (42.9) | |||
| Duke’s stage | ||||||||||
| A | 17 | 12 (70.6) | 5 (29.4) | 0.1350 | 7 (41.2) | 10 (58.8) | 0.3643 | 8 (47.1) | 9 (52.9) | 0.9149 |
| B | 31 | 11 (35.5) | 20 (64.5) | 26 (83.9) | 5 (16.1) | 18 (58.1) | 13 (41.9) | |||
| C | 40 | 18 (45.0) | 22 (55.0) | 25 (62.5) | 15 (37.5) | 17 (42.5) | 23 (57.5) | |||
| D | 14 | 7 (50.0) | 7 (50.0) | 10 (71.4) | 4 (28.6) | 8 (57.1) | 6 (42.9) | |||
Values in bold signify P < 0.05
aP values were calculated using Pearson Chi-square test or Chi-square test for trend
bNumbers inside parentheses are percentages of patients
cAll of these patients with distant metastasis presented with liver metastasis
dThe stage was determined by pathological (p) examination. T1 tumor invading submucosa, T2 tumor invading muscularis propria, T3 tumor penetrating muscularis propria and invading subserosa, T4 tumor invading other organs or structures or perforating visceral peritoneum
Cell lines and cell culture
Colon cancer cell lines, LS174T, Caco-2 and CW2 were purchased from the Shanghai Cell Biology Institutes, Chinese Academy of Science (Shanghai, China), and were cultured in Dulbecco’s modified Eagle’s medium (DMEM, Gibco, USA), containing 10% heat-inactivated fetal bovine serum (FBS), 2 mM l-glutamine, 100 U/ml penicillin and 100 μg/ml streptomycin at 37°C in a humidified incubator supplemented with 5% CO2.
Cytokines and antibodies
Cytokines TNF-α and IFN-γ were purchased from R&D Systems, USA. PE-labeled goat anti-mouse IgG from Immunotech, France. Streptavidin-PE from BD PharMingen, USA. Avidin-PE from Immunotech, France. Matrix metalloproteinase inhibitor (MMPI) from Usbiological, USA. Mouse anti-human B7-H3 monoclonal antibodies, Clone No. 4H7 and 21D4, were established and characterized in our institute. Antibodies included in immunohistochemistry: mouse anti-human CD3 monoclonal antibody (Clone No. SP7, NeoMarkers, CA), rabbit anti human TNF-α polyclonal antibody (Boster Biotechnology Ltd. Corp., China), mouse anti-human B7-H3 monoclonal antibody (Clone No. 4H7, established and characterized in our institute) and horseradish peroxidase (HRP)-labeled goat anti-mouse/rabbit secondary antibody (Dako, Glostrup, Denmark).
Serum samples
For soluble B7-H3 detection, serum samples were collected from 34 CRC patients before surgery by the venipuncture technique. In brief, blood collected by venipuncture was allowed to clot for 20 min at room temperature for serum samples and then centrifuged for 20 min at 666g. Supernatants were collected and stored at −80°C. As controls, we analyzed serum samples from 60 cases of healthy donors without any evidence of cancer history.
Immunohistochemistry procedures
Immunohistochemistry was performed using the Dako EnVisionTM method according to the manufacturer’s instructions. In brief, 3-μm thick consecutive sections were cut by microtome, dewaxed in xylene and rehydrated through graded ethanol solutions. Antigens were retrieved by heating the tissue sections at 100°C for 30 min in citrate solution or EDTA solution when needed. Sections were cooled down and immersed in 0.3% H2O2 solution for 20 min to block endogenous peroxidase activity, and then rinsed in PBS for 5 min, blocked with 5% BSA at room temperature for 20 min, and incubated with primary antibodies against CD3, TNF-α (diluted in 1:100), or B7-H3 (final concentration in use, 10 μg/ml) at 4°C overnight. Negative controls were performed by replacing the specific primary antibody with PBS. After three PBS washes, sections were incubated with secondary antibodies for 30 min at room temperature. Diaminobenzene was used as the chromogen and hematoxylin as the nuclear counterstain. Sections were dehydrated, cleared and mounted.
Evaluation of B7-H3 immunohistochemical staining
Two independent observers who were blinded to the clinicopathological parameters of patients examined the immunohistochemically stained sections. Sections were considered as positive when the tumor cells showed cytoplasmic or membranous B7-H3 immunostaining. The B7-H3 immunostaining intensities were scored according to a scale as Grade 0, negative; Grade 1, weak positive; Grade 2, moderate positive; Grade 3, strong positive. The negative grade represented no tumor cells showing positive immunostaining. For analysis, the B7-H3 immunostaining intensities were classified as follows: the sections scored as Grade 0 and Grade 1 were defined as low expression group, and other sections scored as Grade 2 and Grade 3 were defined as high expression group.
Evaluation of infiltrating T lymphocytes in CRC tissues
Tumor infiltrating T lymphocytes both in tumor stroma and in tumor nest were determined according to the CD3 immunostaining. First, the infiltrating T lymphocytes in tumor stroma were examined at low magnification (×40) and categorized according to density as: Grade 0, scanty; Grade 1, moderate infiltration; Grade 2, abundant infiltration; Grade 3, massive infiltration. The group containing Grade 0 and Grade 1 was defined as low infiltration group, and another group containing Grade 2 and Grade 3 was defined as high infiltration group. Second, the infiltrating T lymphocytes in tumor nest were counted as follows: five areas in tumor nest with the most intense infiltrating T lymphocytes were selected at low magnification (×40), and then the infiltrating T lymphocytes were counted and recorded at high power field (HPF, ×200 magnification). Results from the five areas were averaged and used in the statistical analysis. In the present study, the sections with less than 60 TILs per HPF were defined as low infiltration group, and sections with more than 60 TILs per HPF were defined as high infiltration group. The cut-off value of 60 TILs per HPF for low/high infiltrating assessment in tumor nest was set at the median value of all the sections.
Flow cytometry analysis of membranous B7-H3 expression in CRC cell lines
Cell lines LS174T, Caco-2 and CW-2 were examined for membranous B7-H3 expression using mouse anti-human B7-H3 monoclonal antibody (Clone No. 4H7). Cells were collected after cultivation with or without TNF-α (20 ng/ml) or IFN-γ (50 ng/ml) for 72 h. For flow cytometry analysis, cells (1 × 106) were incubated with the biotinylated mAb B7-H3 (bio-4H7) for 30 min at 4°C and washed. Streptavidin-PE was then added to the cells which were incubated for an additional 30 min. The membranous B7-H3 expression was analyzed by flow cytometry. Meanwhile, the supernatants of cell culture were collected and subjected to soluble B7-H3 measurement by ELISA assay.
ELISA assay
Soluble B7-H3 detection was performed as previously using a sensitive dual monoclonal antibody sandwich enzyme-linked immunosorbent assay (ELISA) kit established in our lab [31].
Statistical analysis
Statistical analyses were performed using GraphPad Prism 4.0 software package (GraphPad Software, Inc., San Diego, USA). Paired or unpaired Student’s t test, or the Pearson Chi-square test was used, where appropriate. P-values less than 0.05 were considered as being statistically significant.
Results
B7-H3 expression in CRC tissues and its correlation to patient’s clinical parameters
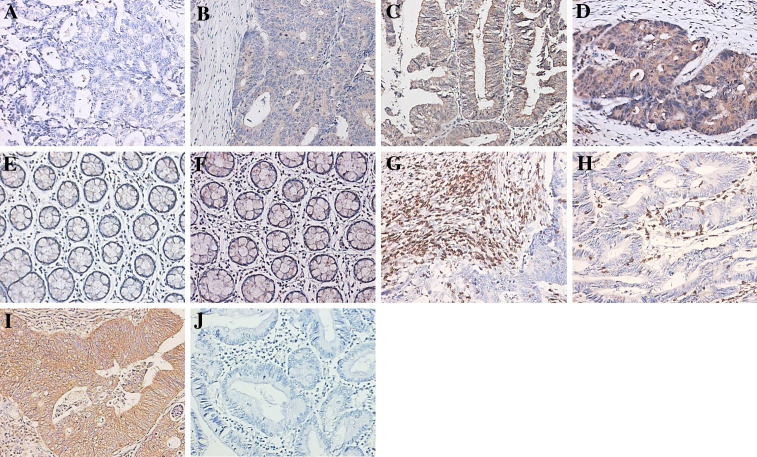
In the present immunohistochemistry study, we characterize B7-H3 expression in resected specimens from 102 CRC patients. Our study showed that the intensity of B7-H3 expression varied and the immunolocalization of B7-H3 molecule was predominantly in the membrane and cytoplasm of colorectal tumor cells. We have found 48 cases of low B7-H3 expression, including 13 of Grade 0 (Fig. 1a) and 35 cases of Grade 1 (Fig. 1b). The other 54 cases were of high B7-H3 expression, including 23 cases of Grade 2 (Fig. 1c) and 31 cases of Grade 3 (Fig. 1d). The B7-H3 expression in CRC tissues positively correlated to patient’s tumor grade (P = 0.0227), suggesting that B7-H3 is involved in cancer progression. B7-H3 expression levels, however, did not correlate to other clinicopathological parameters, such as gender, age, tumor location, tumor size, nodal metastasis, distant metastasis and Duke’s stage (Table 1).
Fig. 1.
B7-H3, CD3 and TNF-α immunostaining. B7-H3 immunostaining in colorectal carcinoma tissues a negative, b weak positive, c moderate positive, d strong positive; B7-H3 immunostaining in normal colorectal tissues e negative, f weak positive; Panel g infiltrating T lymphocytes in tumor stroma; Panel h infiltrating T lymphocytes in tumor nest; Panel i TNF-α immunostaining in colorectal carcinoma; Panel j negative control. Magnifications for all panels ×200
Survey of B7-H3 expression in normal colorectal tissues
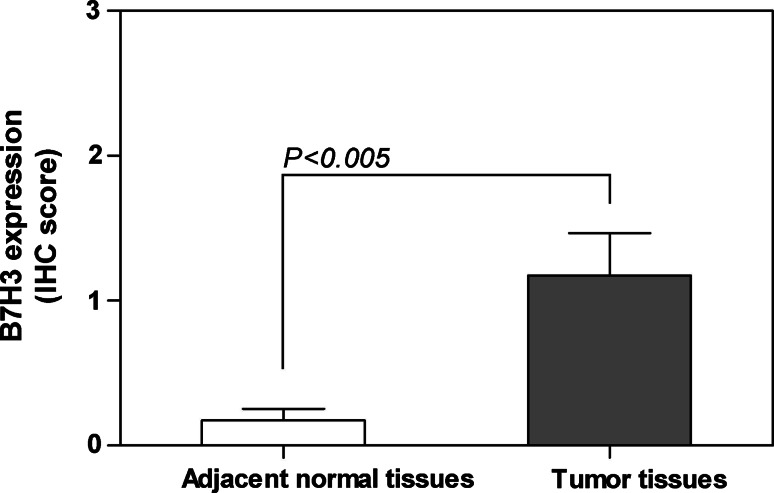
As much as 23 cases of adjacent normal colorectal tissues were resected from surgery and used to survey the B7-H3 expression. Consistent to previous reports [32, 33], we also found B7-H3 was weakly expressed in adjacent normal colorectal tissues (Fig. 1e, none; f, weak positive). Based on the immunohistochemistry scores, we compared the B7-H3 immunostaining intensities between adjacent normal colorectal tissues and tumor tissues from 23 CRC patients, which demonstrated that the B7-H3 expression in tumor tissues was significantly stronger than that in adjacent normal colorectal tissues (P < 0.005, Fig. 2).
Fig. 2.
The B7-H3 expression levels in tumor tissues and in adjacent normal tissues from 23 cases of CRC patients evaluated by immunohistochemical study (P < 0.005)
Survey of soluble B7-H3 in CRC patient’s serum
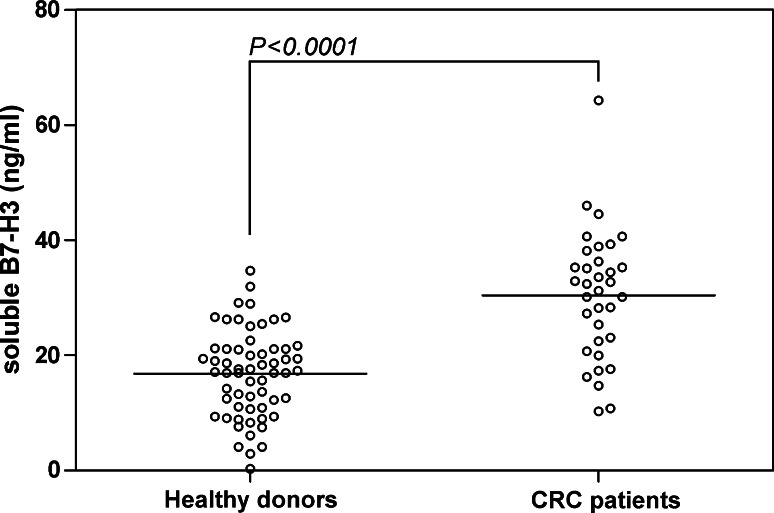
Our previous work has demonstrated that in addition to membranous B7-H3, soluble B7-H3 is present at higher levels in lung cancer patients [35]. We therefore decided to determine whether it is the same case with CRC. Soluble B7-H3 was detected using an ELISA assay as previous [31] in serum from 34 CRC patients and 60 healthy donors. As shown in Fig. 3, although there is quite a level of soluble B7-H3 (16.80 ± 7.49 ng/ml) in normal human serum, the soluble B7-H3 concentration in the serum from CRC patients was significantly increased (30.41 ± 11.14 ng/ml, P < 0.0001).
Fig. 3.
Increased soluble B7-H3 was found in serum from 34 cases of CRC patients (30.41 ± 11.14 ng/ml) in contrast to 60 cases of healthy donors (16.80 ± 7.49 ng/ml) (P < 0.0001)
B7-H3 expression in CRC cell lines
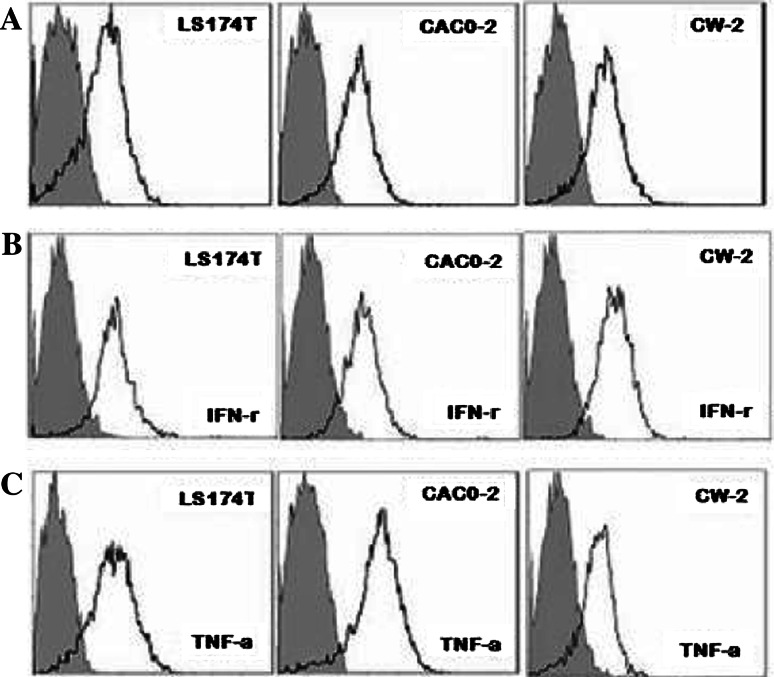
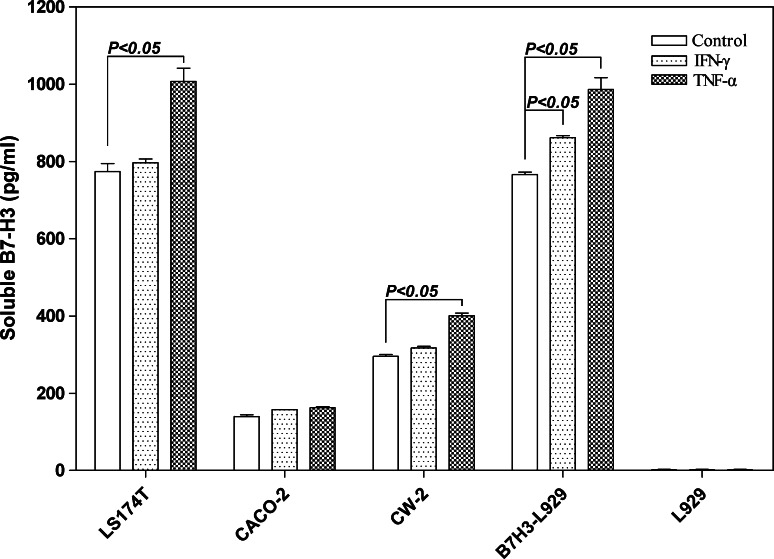
Inflammatory cytokines are well-known to promote carcinogenesis, particularly colon cancer [36]. Our immunohistochemistry study revealed that TNF-α was extensively expressed in colorectal cancer cells (Fig. 1i). We thereby examined whether the inflammatory cytokine TNF-α is involved in the regulation of B7-H3 levels in cancer cells. IFN-γ was shown to induce the expression of B7-H3 in DCs [37] and was therefore included in our study. Flow cytometry analysis demonstrated that three colon cancer cell lines exhibited high expression of membranous B7-H3 (Fig. 3a). The cytokines TNF-α (20 ng/ml) and IFN-γ (50 ng/ml) were individually added into the cell culture to investigate their contribution to membranous/soluble B7-H3 expression. The flow cytometry analysis showed that both TNF-α and IFN-γ exerted no significant influence to the membranous B7-H3 expression (Fig. 4b, c) whereas the B7-H3 transcription could be upregulated by TNF-α (data not shown). Then, the ELISA detection showed that the secretion of soluble B7-H3 in LS174T and CW-2 culture supernatants was significantly increased by TNF-α stimulation but not IFN-γ in contrast to the untreated control (P < 0.05, Fig. 5).
Fig. 4.
Membranous B7-H3 expression in CRC cell lines. a Negative control; b stimulated by 50 ng/ml IFN-γ. c stimulated by 20 ng/ml TNF-α
Fig. 5.
Soluble B7-H3 secretion by CRC cell lines stimulated by inflammatory cytokines IFN-γ and TNF-α, respectively. Increased soluble B7-H3 was detected when the LS174T and CW-2 stimulated by TNF-α at 20 ng/ml for 72 h cultivation in contrast to the negative control (P < 0.05). B7-H3-L929 cell lines were used as positive control
Matrix metalloproteinase (MMP) is required for the production of soluble B7-H3 in CRC cell lines
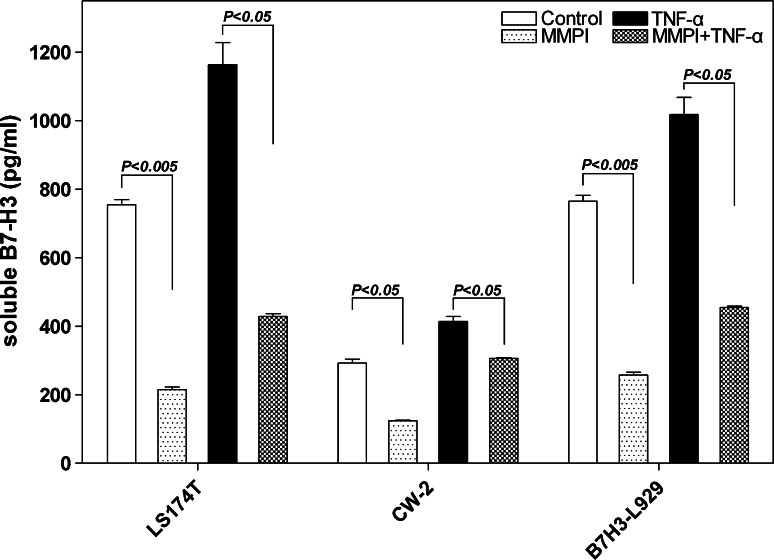
Matrix metalloproteinase is involved in shedding of membranous proteins [38]. We therefore tested the idea that MMP was also involved in shedding B7-H3. A MMP inhibitor (10 μmol/L) was added into the culture supernatants of LS174T, CW-2 and positive control B7-H3-L929 for 72 h cultivation. We found that the MMP inhibitor could significantly decrease the soluble B7-H3 secretion when the cell lines were treated by TNF-α (P < 0.05) or untreated compared with the control (both LS174T and B7-H3-L929 groups: P < 0.005, CW group: P < 0.05) (Fig. 6). Therefore, membrane shedding by MMPs is required for the production of soluble B7-H3 by CRC cells.
Fig. 6.
Increased soluble B7-H3 secretion stimulated by TNF-α could be reduced by MMPI. The MMP inhibitor (10 μmol/L) was added into the culture supernatants of LS174T, CW-2 and B7-H3 transfected cells L929 (positive control). MMPI could significantly decrease the soluble B7-H3 secretion when the cell lines were treated by TNF-α (P < 0.005) or untreated compared with the control (both in LS174T and in B7-H3-L929 groups P < 0.005, in CW group P < 0.05)
B7-H3 expression in relation to infiltrating T lymphocytes in CRC tissues
B7-H3 has been implied to play an immune suppressive role in cancer mainly based on evidence obtained by studying its function in autoimmune diseases [28, 37]. The presence of tumor infiltrating T cells has been used as an indicator for tumor immune surveillance [39]. We therefore examined the number of tumor infiltrating T lymphocytes on specimens from all 102 patients by CD3 immunostaining. We found that the intensity of T cells infiltration in tumor stroma but not in the tumor nest was negatively correlated with tumor grade (P = 0.0410) (Table 1). Moreover, we found that the B7-H3 expression in tumor sections was inversely correlated with the density of infiltrating T lymphocytes both in tumor stroma (P = 0.0354) and in tumor nest (P = 0.0173) (Table 2). This result supports the idea that B7-H3 plays an important role in suppressing immune surveillance of cancer.
Table 2.
Correlation between infiltrating T lymphocytes and B7-H3 expression in CRC tissues
| Infiltrating T lymphocytes in CRC tissues | Cases | B7-H3 expression | χ2 value | P value | |||
|---|---|---|---|---|---|---|---|
| Low | Ratios (%) | High | Ratios (%) | ||||
| In tumor stroma | |||||||
| Low infiltrating | 68 | 27 | 39.7 | 41 | 60.3 | 4.427 | 0.0354 |
| High infiltrating | 34 | 21 | 61.8 | 13 | 38.2 | ||
| In tumor nest | |||||||
| Low infiltrating | 51 | 18 | 35.3 | 33 | 64.7 | 5.667 | 0.0173 |
| High infiltrating | 51 | 30 | 58.8 | 21 | 41.2 | ||
| Total | 102 | ||||||
Values in bold signify P < 0.05
Discussion
In the present study, we have shown that B7-H3 was highly expressed in colorectal cancer cells. Increased B7-H3 expression was found in 54.3% of all 102 CRC patients. B7-H3 expression was significantly correlated to patient’s tumor grade. Furthermore, we have demonstrated that the B7-H3 expression negatively associated with the intensity of infiltrating T lymphocytes both in tumor nest and in tumor stroma. We therefore provided evidence to support the idea that B7-H3 is involved in the suppression of tumor immune surveillance. Moreover, we have found that the cancer-promoting inflammatory cytokine TNF-α increased the B7-H3 transcription and induced the production of soluble B7-H3, providing a potential mechanism for regulation of B7-H3 expression in tumor microenvironment.
Many recent studies have supported a role of B7-H3 in cancer progression. It was reported that B7-H3 was highly expressed in human non-small cell lung cancers, and was significantly correlated to increased risk of lymphnode metastases [24]. Roth et al. [32] evaluated B7-H3 immunoreactivity in more than 300 patients who suffered from prostate cancer and underwent radical prostatectomy, and indicated that increased levels of B7-H3 intensity correlated with worsened clinicopathological features as well as poorer postoperative prognosis. This result was further confirmed by an expanded sample in tissue microarray [33]. B7-H3 immunostaining represented as an additional tool for the differential diagnosis of small round cell tumors and might be useful in identifying neuroblastoma patients at risk of relapse who may take advantage of more careful follow-up [40]. The latest report demonstrated that B7-H3 expression in clear cell renal cell carcinoma was found in both tumor cell and tumor vasculature and represented prognostic implications [34]. Thus, based on previous literatures and our findings in the present study, we concluded that B7-H3 expression might play physiological and pathological role in the oncogenesis and development of CRC.
The exact role of B7-H3 in cancer progression remains elusive. One possible scenario is that B7-H3 is involved in the suppression of tumor immune surveillance and our data provide some experimental evidence in favor of this hypothesis. In the present study, we found that B7-H3 expression in CRCs was inversely correlated to the intensity of infiltrating T lymphocytes, suggesting that one of the most important contributions of B7-H3 expression in this malignancy was to negatively regulate the T lymphocytes infiltration. It is noteworthy that the type, density and location of immune cells in CRC tissues are significantly associated with the early metastatic invasion and the prolonged survival [4, 39] and the presence of Th1 cells is an independent prognostic marker for patient survival [39]. Therefore, our future study will focus on whether the expression of B7-H3 could be a prognostic marker for the survival of CRC patients and examine whether this marker is independent of current cancer staging system.
The underlying mechanism of B7-H3 expression in CRC has not yet been elucidated. In our research, flow cytometry indicated that membranous B7-H3 expression was over-expressed in all three CRC cell lines, and could be regulated by neither TNF-α nor IFN-γ stimulation. However, we found that TNF-α significantly increases the soluble B7-H3 secretion in CRC cell lines LS174T and CW-2. It has been demonstrated that inflammatory cytokines were involved in the development of colorectal inflammatory or carcinogenic lesions, especially TNF-α, which was an essential mediator of the initiation and progression of colitis-associated colorectal carcinogenesis [41]. These results suggested that the inflammatory cytokine TNF-α might act as an important modulator in the induction of aberrant B7-H3 expression in the tumor environment of colorectal carcinoma.
We have previously demonstrated the soluble B7-H3 is released from monocytes, dendritic cells and activated T cells, and is available to be detected using the ELISA assay we established in our lab [31]. Moreover, we newly identified that soluble B7-H3 molecule in serum from patients with NSCLC were significantly higher than those in patients with other pulmonary diseases or those in healthy volunteers [35], which suggested that circulating B7-H3 could be developed as a promising serum biomarker to improve NSCLC diagnosis and prognostic assessment. Therefore, it is necessary for us to design a study and collect a large and well-characterized patient samples to investigate the diagnostic value of circulating B7-H3 levels in CRC patients.
In conclusion, our present findings indicate that the costimulatory molecule B7-H3 plays an important role in CRC progression by means of membranous and soluble forms, and might be involved in the negative regulation of T lymphocytes-mediated tumor immune response. Moreover, TNF-α is an important modulator in soluble B7-H3 secretion. In the future, a precise mechanism of B7-H3 expression regulation in tumor environment, overall knowledge of its clinical implications and targeted therapeutic interventions in CRC warrant further investigations.
Acknowledgments
We thank senior pathologists Chang-qing Lu, Min Tan, Yuan-dong Chen and Prof. Tong-yu Chen (Department of Pathology, The Third Affiliated Hospital of Suzhou University, Jiangsu Changzhou 213003, China) for their excellent expert suggestions and technical assistances. We also thank Dr. Jeff Kovacs and Ms. Christen Shiber (Department of Immunology, University of Pittsburgh) for editing the manuscript. This work was supported by grants from the Major State Basic Research Development Program of China (973 Program: CB51003), the National Natural Science Foundation of China (No. 30801023 and No. 30930085), and the advanced program of Commission of Technology and Industry for National Defense (A3820060130).
Conflict of interest statement
The authors declare that they have no competing interests to this paper.
Footnotes
J. Sun and L. Chen contributed equally to this work.
References
- 1.Weitz J, Koch M, Debus J, Hohler T, Galle PR, Buchler MW. Colorectal cancer. Lancet. 2005;365(9454):153–165. doi: 10.1016/S0140-6736(05)17706-X. [DOI] [PubMed] [Google Scholar]
- 2.Dong Z, Qiao Y, Li L, Chen Y, Wang R, Lei T. Report of Chinese cancer control strategy. J Chin Cancer Res. 2002;11(6):250–260. [Google Scholar]
- 3.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420(6917):860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353(25):2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 5.Bolton JS, Fuhrman GM. Survival after resection of multiple bilobar hepatic metastases from colorectal carcinoma. Ann Surg. 2000;231(5):743–751. doi: 10.1097/00000658-200005000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vogelsang H, Haas S, Hierholzer C, Berger U, Siewert JR, Prauer H. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91(8):1066–1071. doi: 10.1002/bjs.4602. [DOI] [PubMed] [Google Scholar]
- 7.Sharpe AH, Freeman GJ. The B7-CD28 superfamily. Nat Rev Immunol. 2002;2(2):116–126. doi: 10.1038/nri727. [DOI] [PubMed] [Google Scholar]
- 8.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 9.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 10.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, Iwai Y, Long AJ, Brown JA, Nunes R, Greenfield EA, Bourque K, Boussiotis VA, Carter LL, Carreno BM, Malenkovich N, Nishimura H, Okazaki T, Honjo T, Sharpe AH, Freeman GJ. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–268. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 11.Yoshinaga SK, Whoriskey JS, Khare SD, Sarmiento U, Guo J, Horan T, Shih G, Zhang M, Coccia MA, Kohno T, Tafuri-Bladt A, Brankow D, Campbell P, Chang D, Chiu L, Dai T, Duncan G, Elliott GS, Hui A, McCabe SM, Scully S, Shahinian A, Shaklee CL, Van G, Mak TW, Senaldi G. T-cell co-stimulation through B7RP-1 and ICOS. Nature. 1999;402(6763):827–832. doi: 10.1038/45582. [DOI] [PubMed] [Google Scholar]
- 12.Chapoval AI, Ni J, Lau JS, Wilcox RA, Flies DB, Liu D, Dong H, Sica GL, Zhu G, Tamada K, Chen L. B7-H3: a costimulatory molecule for T cell activation and IFN-gamma production. Nat Immunol. 2001;2(3):269–274. doi: 10.1038/85339. [DOI] [PubMed] [Google Scholar]
- 13.Sica GL, Choi IH, Zhu G, Tamada K, Wang SD, Tamura H, Chapoval AI, Flies DB, Bajorath J, Chen L. B7-H4, a molecule of the B7 family, negatively regulates T cell immunity. Immunity. 2003;18(6):849–861. doi: 10.1016/S1074-7613(03)00152-3. [DOI] [PubMed] [Google Scholar]
- 14.Ohigashi Y, Sho M, Yamada Y, Tsurui Y, Hamada K, Ikeda N, Mizuno T, Yoriki R, Kashizuka H, Yane K, Tsushima F, Otsuki N, Yagita H, Azuma M, Nakajima Y. Clinical significance of programmed death-1 ligand-1 and programmed death-1 ligand-2 expression in human esophageal cancer. Clin Cancer Res. 2005;11(8):2947–2953. doi: 10.1158/1078-0432.CCR-04-1469. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Xu K, Wu C, Wang Y, Hu Y, Zhu Y, Chen Y, Shi Q, Yu G, Zhang X. PD-L1 expression analysis in gastric carcinoma tissue and blocking of tumor-associated PD-L1 signaling by two functional monoclonal antibodies. Tissue Antigens. 2007;69(1):19–27. doi: 10.1111/j.1399-0039.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 16.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–5100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 17.Hamanishi J, Mandai M, Iwasaki M, Okazaki T, Tanaka Y, Yamaguchi K, Higuchi T, Yagi H, Takakura K, Minato N, Honjo T, Fujii S. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci USA. 2007;104(9):3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ghebeh H, Mohammed S, Al-Omair A, Qattan A, Lehe C, Al-Qudaihi G, Elkum N, Alshabanah M, Bin Amer S, Tulbah A, Ajarim D, Al-Tweigeri T, Dermime S. The B7–H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8(3):190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kryczek I, Wei S, Zhu G, Myers L, Mottram P, Cheng P, Chen L, Coukos G, Zou W. Relationship between B7-H4, regulatory T cells, and patient outcome in human ovarian carcinoma. Cancer Res. 2007;67(18):8900–8905. doi: 10.1158/0008-5472.CAN-07-1866. [DOI] [PubMed] [Google Scholar]
- 20.Krambeck AE, Thompson RH, Dong H, Lohse CM, Park ES, Kuntz SM, Leibovich BC, Blute ML, Cheville JC, Kwon ED. B7-H4 expression in renal cell carcinoma and tumor vasculature: associations with cancer progression and survival. Proc Natl Acad Sci USA. 2006;103(27):10391–10396. doi: 10.1073/pnas.0600937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tringler B, Zhuo S, Pilkington G, Torkko KC, Singh M, Lucia MS, Heinz DE, Papkoff J, Shroyer KR. B7-h4 is highly expressed in ductal and lobular breast cancer. Clin Cancer Res. 2005;11(5):1842–1848. doi: 10.1158/1078-0432.CCR-04-1658. [DOI] [PubMed] [Google Scholar]
- 22.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, Nakamura S, Enomoto K, Yagita H, Azuma M, Nakajima Y. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–2157. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 23.Zou W, Chen L. Inhibitory B7-family molecules in the tumour microenvironment. Nat Rev Immunol. 2008;8(6):467–477. doi: 10.1038/nri2326. [DOI] [PubMed] [Google Scholar]
- 24.Sun Y, Wang Y, Zhao J, Gu M, Giscombe R, Lefvert AK, Wang X. B7-H3 and B7-H4 expression in non-small-cell lung cancer. Lung Cancer. 2006;53(2):143–151. doi: 10.1016/j.lungcan.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 25.Ghebeh H, Barhoush E, Tulbah A, Elkum N, Al-Tweigeri T, Dermime S. FOXP3+ Tregs and B7-H1+/PD-1+ T lymphocytes co-infiltrate the tumor tissues of high-risk breast cancer patients: implication for immunotherapy. BMC Cancer. 2008;8:57. doi: 10.1186/1471-2407-8-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gao Q, Wang XY, Qiu SJ, Yamato I, Sho M, Nakajima Y, Zhou J, Li BZ, Shi YH, Xiao YS, Xu Y, Fan J. Overexpression of PD-L1 significantly associates with tumor aggressiveness and postoperative recurrence in human hepatocellular carcinoma. Clin Cancer Res. 2009;15(3):971–979. doi: 10.1158/1078-0432.CCR-08-1608. [DOI] [PubMed] [Google Scholar]
- 27.Hashiguchi M, Kobori H, Ritprajak P, Kamimura Y, Kozono H, Azuma M. Triggering receptor expressed on myeloid cell-like transcript 2 (TLT-2) is a counter-receptor for B7-H3 and enhances T cell responses. Proc Natl Acad Sci USA. 2008;105(30):10495–10500. doi: 10.1073/pnas.0802423105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Prasad DV, Nguyen T, Li Z, Yang Y, Duong J, Wang Y, Dong C. Murine B7-H3 is a negative regulator of T cells. J Immunol. 2004;173(4):2500–2506. doi: 10.4049/jimmunol.173.4.2500. [DOI] [PubMed] [Google Scholar]
- 29.Castriconi R, Dondero A, Augugliaro R, Cantoni C, Carnemolla B, Sementa AR, Negri F, Conte R, Corrias MV, Moretta L, Moretta A, Bottino C. Identification of 4Ig-B7-H3 as a neuroblastoma-associated molecule that exerts a protective role from an NK cell-mediated lysis. Proc Natl Acad Sci USA. 2004;101(34):12640–12645. doi: 10.1073/pnas.0405025101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chavin G, Sheinin Y, Crispen PL, Boorjian SA, Roth TJ, Rangel L, Blute ML, Sebo TJ, Tindall DJ, Kwon ED, Karnes RJ. Expression of immunosuppresive B7–H3 ligand by hormone-treated prostate cancer tumors and metastases. Clin Cancer Res. 2009;15(6):2174–2180. doi: 10.1158/1078-0432.CCR-08-2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, Zhang X. Soluble CD276 (B7-H3) is released from monocytes, dendritic cells and activated T cells and is detectable in normal human serum. Immunology. 2008;123(4):538–546. doi: 10.1111/j.1365-2567.2007.02723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roth TJ, Sheinin Y, Lohse CM, Kuntz SM, Frigola X, Inman BA, Krambeck AE, McKenney ME, Karnes RJ, Blute ML, Cheville JC, Sebo TJ, Kwon ED. B7–h3 ligand expression by prostate cancer: a novel marker of prognosis and potential target for therapy. Cancer Res. 2007;67(16):7893–7900. doi: 10.1158/0008-5472.CAN-07-1068. [DOI] [PubMed] [Google Scholar]
- 33.Zang X, Thompson RH, Al-Ahmadie HA, Serio AM, Reuter VE, Eastham JA, Scardino PT, Sharma P, Allison JP. B7-H3 and B7x are highly expressed in human prostate cancer and associated with disease spread and poor outcome. Proc Natl Acad Sci USA. 2007;104(49):19458–19463. doi: 10.1073/pnas.0709802104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Crispen PL, Sheinin Y, Roth TJ, Lohse CM, Kuntz SM, Frigola X, Thompson RH, Boorjian SA, Dong H, Leibovich BC, Blute ML, Kwon ED. Tumor cell and tumor vasculature expression of B7-H3 predict survival in clear cell renal cell carcinoma. Clin Cancer Res. 2008;14(16):5150–5157. doi: 10.1158/1078-0432.CCR-08-0536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang G, Xu Y, Lu X, Huang H, Zhou Y, Lu B, Zhang X. Diagnosis value of serum B7-H3 expression in non-small cell lung cancer. Lung Cancer. 2009;66(2):245–249. doi: 10.1016/j.lungcan.2009.01.017. [DOI] [PubMed] [Google Scholar]
- 36.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 37.Suh WK, Gajewska BU, Okada H, Gronski MA, Bertram EM, Dawicki W, Duncan GS, Bukczynski J, Plyte S, Elia A, Wakeham A, Itie A, Chung S, Da Costa J, Arya S, Horan T, Campbell P, Gaida K, Ohashi PS, Watts TH, Yoshinaga SK, Bray MR, Jordana M, Mak TW. The B7 family member B7-H3 preferentially down-regulates T helper type 1-mediated immune responses. Nat Immunol. 2003;4(9):899–906. doi: 10.1038/ni967. [DOI] [PubMed] [Google Scholar]
- 38.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2(3):161–174. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 39.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313(5795):1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 40.Gregorio A, Corrias MV, Castriconi R, Dondero A, Mosconi M, Gambini C, Moretta A, Moretta L, Bottino C. Small round blue cell tumours: diagnostic and prognostic usefulness of the expression of B7-H3 surface molecule. Histopathology. 2008;53(1):73–80. doi: 10.1111/j.1365-2559.2008.03070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res. 2008;68(1):323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]