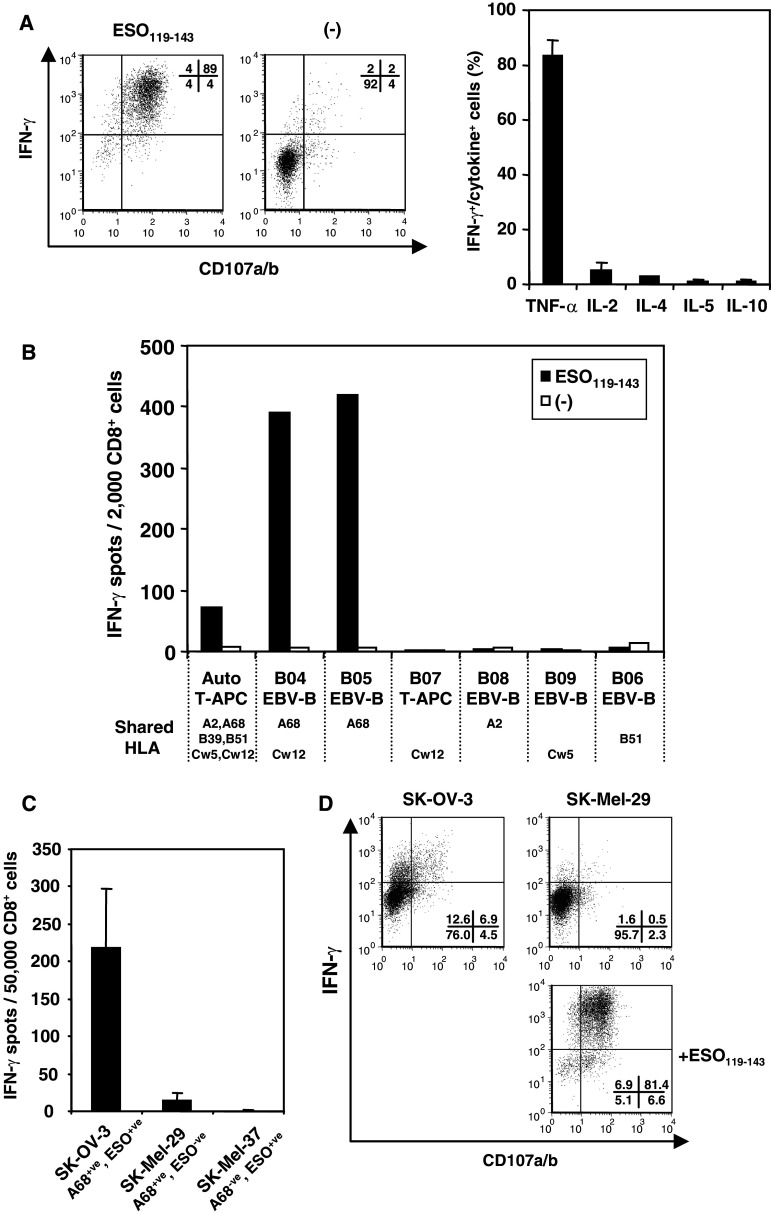
Fig. 2.
Determination of HLA restriction for CTL clone cells obtained from B01 patient. a IFN-γ and CD107 expression by B01 clone was examined in intracellular staining against autologous T-APC pulsed with or without ESO119–143 (left). Various cytokine productions were examined by intracellular staining using anti-TNF-α, anti-IL-2, anti-IL-4, anti-IL-5 and anti-IL-10 mAbs, respectively (right). b B01 clone was stimulated with partially HLA-matched T-APC or EBV-B cells pulsed with or without ESO119–143 peptide. IFN-γ productions from CTL clone were examined by ELISPOT assay. Only shared HLA molecules between the patient and other APCs are represented under the cell names. c The response of B01 clone against HLA-matched tumor cell lines were tested by ELISPOT assay. SK-OV-3 and SK-MEL-37 but not SK-MEL-29 express NY-ESO-1 antigen. d IFN-γ and CD107 expression of B01 clone against HLA-A68+ve tumor cell lines were tested by intracellular staining. For this assay, CTL clone was cultured under the combination of 10 U/ml IL-2, 10 ng/ml IL-15 and 10 U/ml IL-12 for 5 days, and then, the response against tumor cell lines were tested