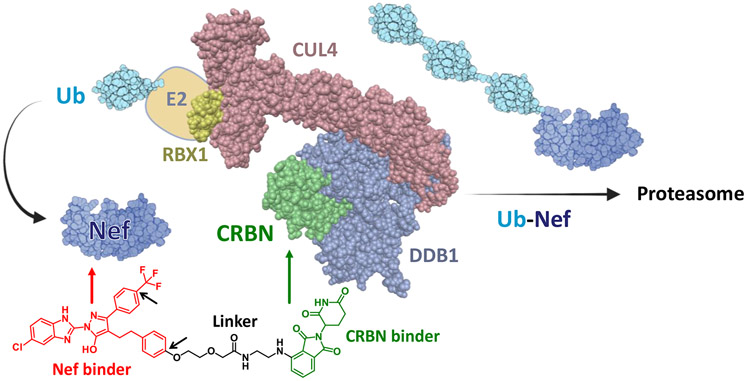
Figure 1. Targeted degradation of HIV-1 Nef by a CRBN-based PROTAC.
The Cereblon (CRBN) ubiquitin E3 ligase complex (left) is a large multiprotein structure composed of RING-box protein 1 (RBX1), Cullin4 (CUL4), DNA damage binding protein 1 (DDB1), CRBN and an E2 subunit conjugated to ubiquitin (Ub). Heterobifunctional Nef PROTACs promote formation of a ternary complex between the HIV-1 Nef protein using existing hydroxypyrazole Nef-binding compounds (red) and the CRBN E3 complex via a CRBN ligand (exemplified by thalidomide, green). Ternary complex formation induces polyubiquitination of Nef and subsequent proteasomal degradation. The Nef PROTAC shown is analog FC-13182; favored positions for linker attachment on the Nef-binding moiety are indicated by the short black arrows. This model was produced using BioRender and structural coordinates for ubiquitin (PDB 1D3Z, NMR), HIV-1 Nef (PDB: 6B72, crystal) and an E3 ligase complex (PDB: 2HYE, crystal).