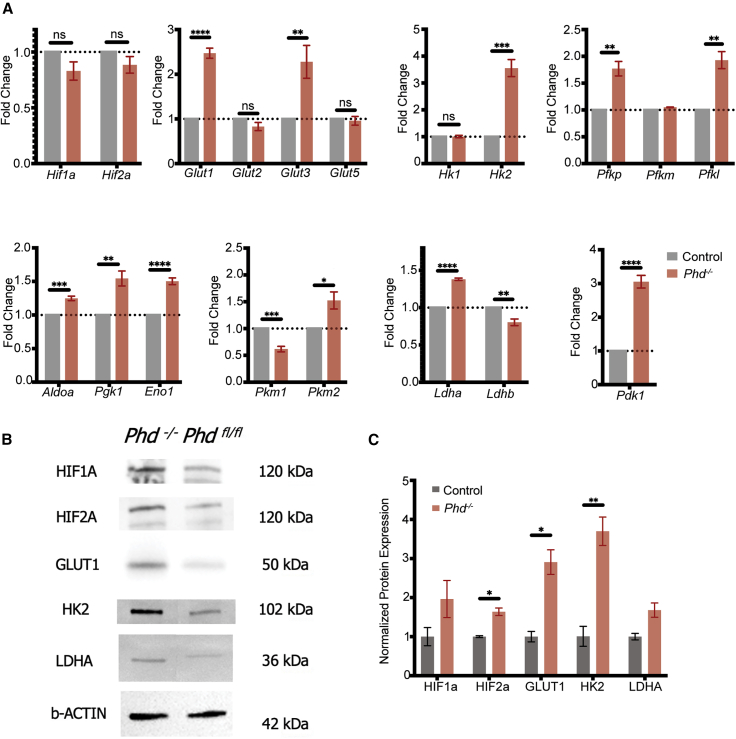
Figure 2.
PHD deficiency up-regulates key regulators of glycolytic metabolism in photoreceptors
Experimental (PHD−/−;Pde6βH620Q/H620Q;Pde6γCreERT2/+) and control (PHDFL/FL;Pde6βH620Q/H620Q;Pde6γCreERT2/+) mice were treated with tamoxifen or sham solution, respectively, for three consecutive days (P8, P9, and P10). Mice were sacrificed at postnatal 3 weeks, and retinas were collected and snap-frozen in liquid nitrogen until further processing.
(A) mRNA expression of Hif1a and Hif2a and the downstream glycolytic targets (Glut1, Glut2, Glut3, Glut5, Hk1, Hk2, Pfkp, Pfkm, Pfkl, Aldoa, Pgk1, Eno1, Pkm1, 2, Ldha, Ldhb, and Pdk1) were quantified to assess changes before and after PHD was ablated. β-Actin was used as the internal control. Error bars indicate SEM. ∗p ≤ 0.05, ∗∗p ≤ 0.01, ∗∗∗p ≤ 0.001, ∗∗∗∗p ≤ 0.0001; n = 4–5 per group.
(B) Representative immunoblots of glycolytic metabolism enzymes and regulators in the retinas of treated and untreated mice at P21 (before the onset of degeneration) to detect HIF1A, HIF2A, GLUT1, HK2, and LDHA protein levels. β-Actin was used as a loading control. Membrane was stripped and reprobed for all targets.
(C) Quantitative analysis of protein levels shown in (B). Error bars indicate SEM. ∗p ≤ 0.05, ∗∗p ≤ 0.01; n = 3 per group.