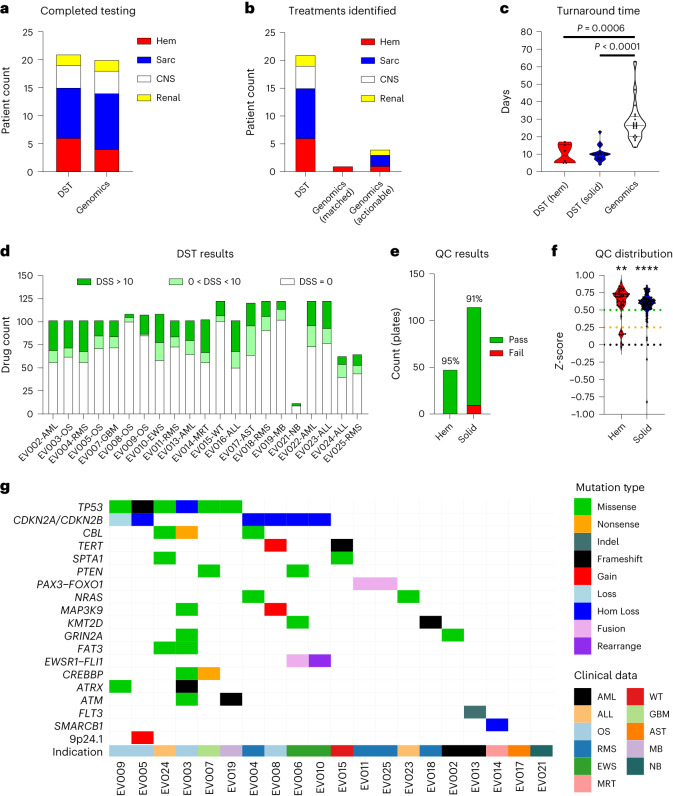
Fig. 3. FPM workflow is feasible and actionable in a clinically relevant timeframe.
a, Results returned from patient sample testing through DST and genomic profiling, distributed by cancer type. CNS, central nervous system; Hem, hematological; Sarc, sarcoma. b, Distribution of patients with reported therapeutic options identified through DST, identified by genomics as an approved therapy matching the patient’s cancer type (Matched) and identified by genomics as an approved therapy in other cancer types (Actionable). c, Distribution of turnaround time in days for DST of hematological cancer samples and solid cancer samples, as well as UCSF500 genomics panel assays. P values determined by adjusted Kruskal–Wallis test (P < 0.0001). d, Distribution of single agent DSS for each patient (ineffective, DSS = 0 (white); moderately effective, 0 < DSS ≤ 10 (light green); effective, DSS > 10 (dark green)). e, Number and percent of DST plates that passed quality control analysis for hematological and solid cancers. QC, quality control. f, Z-prime scores of quality control from DST plates for hematological and solid cancers. P values determined by two-sided one-sample Wilcoxon tests. Hem, P = 0.0045; solid, P = 0.00001. **P < 0.01, ****P < 0.0001. g, Genomic landscape of variants identified through genomic tumor panel profiling using UCSF500. Genes with alterations in two or more patient samples or alterations with matched therapies are reported. Hom, homozygous.