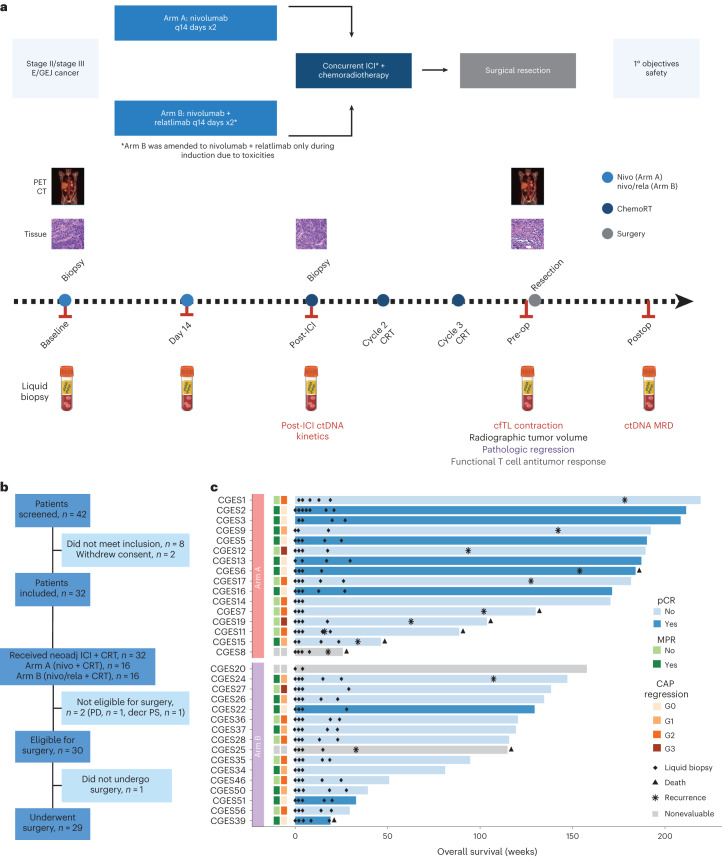
Fig. 1. Clinical trial schema, CONSORT flow diagram and patient characteristics.
a, Patients with resectable clinical stage II/stage III distal E/GEJ adenocarcinoma or SCC were consecutively enrolled in the following two treatment cohorts: nivolumab every 2 weeks for two induction cycles then three additional doses given concurrently with chemoradiation (Arm A) or nivolumab and relatlimab every 2 weeks according to the same schedule (Arm B). Patients were enrolled in Arm B after safety and feasibility objectives were met in Arm A. The primary endpoint of the trial was safety; the secondary endpoint was feasibility; exploratory endpoints included OS, RFS, MPR and pCR rates and biomarker analyses. Baseline CT and PET/CT scans were obtained before the first dose of neoadjuvant treatment, and PET/CT was obtained after completion of neoadjuvant treatment (presurgery). Tumor samples were collected at baseline, after two cycles of induction immunotherapy, and at the time of surgery. Serial blood samples were collected at baseline, start of cycle 2, start of cycle 3, before surgery and within 3–12 weeks after surgery. b, CONSORT flow diagram depicting patient disposition as follows: of the 42 patients screened, 8 did not meet inclusion criteria and 2 withdrew consent. The remaining 32 patients were enrolled in the study; 2 patients were not eligible for surgery (1 patient because of disease progression—PD—and 1 patient because of declining performance status related to CRT). Of the 30 patients eligible for surgery, 1 patient elected not to undergo surgery and the remaining 29 patients underwent Ivor Lewis esophagectomy. c, Swimmer’s plot depicting pCR, MPR, CAP tumor regression, recurrence, death and OS, together with blood collection for liquid biopsy analyses for each patient. Patients are grouped by trial arm and ordered by OS within each arm. The bar color indicates pCR. CONSORT, Consolidated Standards of Reporting Trials; ICI, immune checkpoint inhibitors; neoadj, neoadjuvant; PD, progressive disease; cfTL, cell-free tumor load.