Fig. 4.
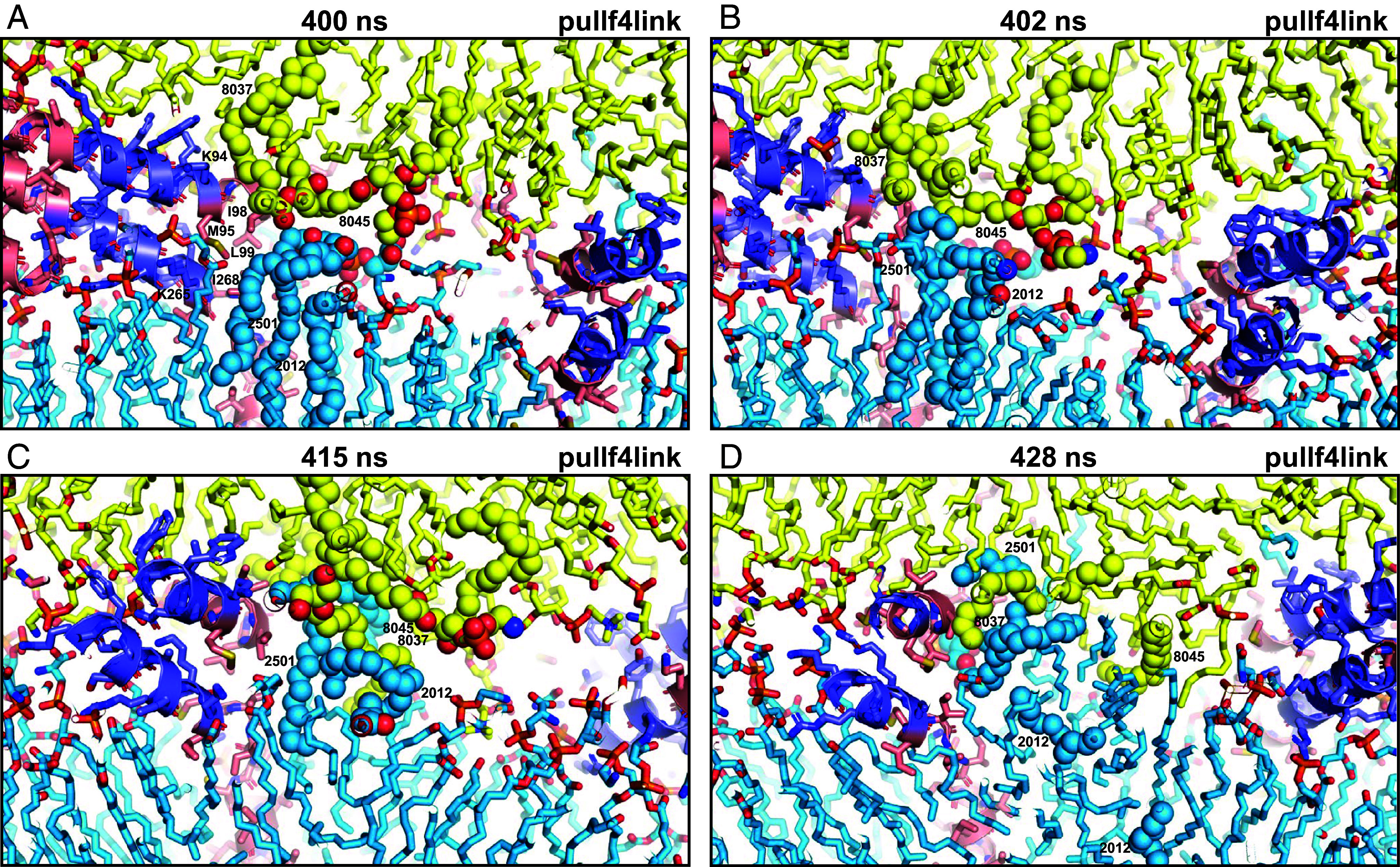
Snapshots taken at the indicated time points of the pullf4link simulation showing the formation of a small hydrophobic nucleus at the polar interface. The diagrams illustrate how there were no hydrophobic contacts in this region of the polar interface between the two bilayers at 400 ns (A). Acyl chains of polyunsaturated lipids from the two bilayers came into contact at the polar interface at 402 ns (B), when they came out of the bilayers due to fluctuations next to the methylene groups of lysine side chains from the linkers and hydrophobic side chains from the TM regions [labeled with the single letter amino acid residue abbreviation and residue number in (A)]. Additional contacts kept forming next to these residues (C) and facilitated the formation of hydrophobic acyl chain contacts, forming a small hydrophobic nucleus (D). Lipids and proteins are shown as stick models, and SNARE complexes are in addition represented by ribbon diagrams. The color code is the same as in Fig. 1. The positions of SDPE2012, SDPS2501, SAPE8037, and SAPE8045 (shown as spheres), which were involved in the initial contacts, are indicated. Note that parts of these lipids cannot be seen in the slice of the system shown. This is why the two acyl chains of SDPE2012 look disconnected in panel (D).
