Abstract
Purpose
Acute ischemic stroke (AIS) stands as the primary cause of mortality and extended disability globally. While prior studies have examined the connection between stroke and local weather, they have produced conflicting results. Our goal was to examine the correlation between temperature and functional prognosis in patients with large vessel occlusion (LVO) undergoing endovascular therapy (EVT).
Patients and methods
This study included a total of 1809 patients. Temperatures from stroke onset to groin puncture were categorized into Cold (10th percentile of temperature), Cool (10th–50th percentile of temperature), Warm (50th–90th percentile of temperature), and Hot (90th percentile of temperature) groups. The primary efficacy result was the modified Rankin Scale (mRS) score at 90 days. Safety outcomes included mortality, symptomatic intracranial hemorrhage (sICH) and complications after cerebral infarction.
Results
The primary efficacy results demonstrated a statistical enhancement in functional outcomes at 90 days for patients in the Warm group compared to the Cold group (adjusted common odds ratio [OR]: 1.386; 95% confidence interval [CI]: 1.024–1.878, P=0.035). Secondary efficacy results showed that temperature was associated with a higher rate of 90-day functional independence (adjusted OR: 1.016; 95% CI: 1.004–1.029; P=0.009), which was higher in the Warm group compared with patients in the Cold group (adjusted OR: 1.646; 95% CI: 1.107–2.448, P=0.014). There were no significant differences between groups in terms of sICH, 90-day mortality, and post-infarction complications.
Conclusion
Compared with Cold temperature, Warm temperature is associated with better functional outcomes and reduced mortality risk without increasing the risk of sICH.
Keywords: acute ischemic stroke, ambient temperature, large-vessel occlusion, endovascular treatment
Introduction
According to WHO statistics, stroke is the primary reason for fatalities and enduring impairment globally. Endovascular treatment (EVT) for patients with acute large vessel occlusion (LVO) is currently recognized as the standard medical care.1 The Lancet Neurology published a systematic analysis for the Global Burden of Disease Study 2019 shows that 87% of strokes can be attributed to 19 risk factors, including smoking, high body mass index, environmental particulate matter pollution, high systolic blood pressure, high salt diet, high LDL, renal insufficiency, high fasting glucose, high or low ambient temperature, low physical activity, etc.2 There is ongoing debate about the impact of ambient temperature on stroke.
Numerous studies have confirmed that colder temperatures are linked to an increased risk of stroke.3–9 Other researchers found that increased average temperatures were linked to a greater occurrence of ischemic stroke.10–12 Several studies have stated that they did not observe a significant correlation between outdoor temperature and ischemic stroke.13–16 Conversely, another meta-analysis suggested that both low and high temperatures can elevate the risk of stroke mortality.13 Despite numerous studies examining the relationship between climate factors and the risk of stroke, there is still no clear consensus on the associations between ambient temperatures and stroke prognosis.
We utilized a unified nationwide database from three studies to assess whether ambient temperatures could affect functional outcomes of patients with endovascular treatment due to acute large vessel occlusion.
Materials and Methods
Patient Selection
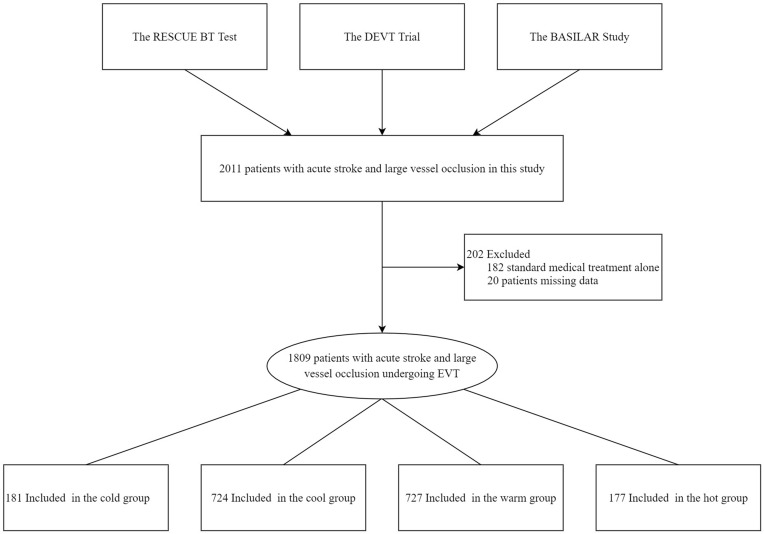
Data from three EVT studies of patients with AIS due to LVO were included: the RESCUE BT test is a multicenter, randomized, placebo-controlled, double-blind trial conducted in approximately 50 centers in China, which compares endovascular treatment with and without tirofiban for stroke patients with large vessel occlusion. Additionally, the DEVT trial is an open-label, multicenter, randomized controlled trial focusing on acute ischemic stroke (AIS) caused by anterior large vessel occlusion (LVO) at 33 stroke centers in China. Lastly, the BASILAR study is a nationwide registry for acute basilar artery occlusion, involving 47 stroke centers in China (Figure 1). The above three study protocols were approved by Xinqiao Ethics Committee and the sub-centers’ ethics committees (Supplement Appendix eTables 1–3) and complies with the Declaration of Helsinki. Patients who were eligible or their legal representatives provided informed consent before participating in the above study.
Figure 1.
Flow of Patients in a study of the effect of temperature for patients with Endovascular Treatment. This study included 2011 patients with acute stroke and large vessel occlusion. However, 202 patients were excluded for different reasons: 182 received only standard medical treatment, while the remaining 20 patients had missing essential data. Therefore, the final sample size consisted of 1809 patients who underwent treatment for large vessel occlusion.
Patients aged 18 years or older with confirmed LVO of the posterior circulation or anterior circulation from head digital subtraction angiography, magnetic resonance angiography (MRA), or computer tomography angiography (CTA), and who received EVTs within 24 hours of the estimated time of LVO for the anterior circulation, or within 72 hours for the posterior circulation, were included. Exclusion criteria comprised a premorbid modified Rankin Scale (mRS) of 3 or more, cerebral hemorrhage revealed by CTA or MRA, and severe liver, renal, heart, lung dysfunction.
Methods
In this study, the minimum temperature to the 10th percentile of the temperature distribution was noted as Cold group, the 10th to 50th percentile of the temperature distribution was noted as Cool group, the 50th to 90th percentile of the temperature distribution was noted as Warm group, and the 90th percentile of the temperature distribution to the maximum temperature was noted as Hot group. Temperature was defined as the mean temperature distribution from stroke onset to groin puncture. The primary efficacy result was the modified Rankin Scale (mRS) score at 90 days. The secondary efficacy result included favorable functional outcomes, functional independence, and excellent outcome (defined as an mRS score of 3 or less) at 90 days. Safety outcomes included symptomatic intracranial hemorrhage (sICH) within 48 hours, mortality within 90 days and complications after cerebral infarction (including cerebral hernia, decompressive craniectomy, pneumonia, respiratory failure, circulatory failure, venous thrombosis, and death in hospital).
Temperatures Acquisition
All patients who met the inclusion criteria had air temperatures collected from stroke onset to groin puncture. We obtained data on temperature from the Meteorological public applet (http://www.meteologix.com/cn).
Data Collection and Assessment
The combined database was used to extract demographic information and baseline clinical characteristics of all eligible patients. This encompasses age, gender, and medical history such as hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, atrial fibrillation, and smoking. Additionally, blood pressure at admission and National Institutes of Health Stroke Scale (NIHSS) scores at admission were recorded. Stroke subtypes were categorized based on the Trial of Org 10172 in Acute Stroke (TOAST). The time from stroke onset to groin puncture and revascularization was documented, with stroke onset defined as the last time the patient was observed in normal state. Baseline ischemic injury in the anterior and posterior circulation were evaluated using Alberta Stroke Program Early Computed Tomography (ASPECT) score. The quality of reperfusion was assessed using the modified Thrombolysis in Cerebral Infarction (mTICI) score on the final angiogram. Collateral vessel status was assessed using the American Society of Interventional and Therapeutic Neuroradiology/Society/Society of Interventional Radiology (ASITN/SIR) collateral vessel grading system.
Clinical Outcomes
The primary measure of effectiveness outcome was the mRS score at 90 days, which ranged from 0 [no symptoms] to 6 [death]. Neurologists who were blinded to the treatment information conducted follow-up examinations through outpatient visits or phone calls at participating centers. Secondary measure of effectiveness included a favorable functional outcome (defined as an mRS score of 0–3), functional independence (mRS score of 0–2), and an excellent outcome (mRS score of 0–1). Safety measures included symptomatic intracranial hemorrhage (sICH) within 48 h, complication after cerebral infarction and mortality within 3 months of EVT. The assessment of sICH was conducted using follow-up CT or MRI in accordance with the Heidelberg Bleeding Classification.
Statistical Analysis
We divided the average temperature distribution from stroke onset to groin puncture into four subgroups. The Chi-squared test was employed for analyzing categorical variables, while the Mann–Whitney U-test was utilized for comparing continuous variables. These statistical tests were applied to assess and compare baseline characteristics and outcomes across the four subgroups in the study. Multivariable ordinal logistic regression was used to analyze the shift in the mRS score towards improvement by one score between four subgroups. The analysis was adjusted for several variables, including age, sex, smoking, hypertension, hyperlipidemia, diabetes mellitus, ischemic stroke, atrial fibrillation, baseline NIHSS, TOAST classification, time from stroke onset to revascularization, general anesthesia, occlusion sites, intravenous thrombolysis (IVT), and mTICI score. For other, binary logistic regression was performed to analyze other dichotomized outcomes, and the same confounding factors were adjusted.
We conducted a statistical analysis using SPSS (version 23.0; IBM SPSS Statistics) and created figures using Microsoft Office Excel 2019. A significance level of P < 0.05 was set, and all hypothesis tests were two-sided.
Results
Baseline Characteristics
A total of 1809 patients were included in this study. The median (interquartile range) age was 67 (57–74) years, 1159 (64.1%) of the patients were men, and the baseline NIHSS score was 17 (13–23). The rate of IVT was 14.0%, and 87.3% (1575/1805) of patients achieved successful reperfusion (mTICI 2b–3). The patients were categorized into four groups according to their temperature: Cold group (T≤6.47°C), Cool group (T=6.48–19.78°C), Warm group (T=19.79–29.83°C), and Hot group (T≥29.84°C). The distribution of patients in these groups was as follows: 181 (10.0%) in the Cold group, 724 (40.0%) in the Cool group, 727 (40.2%) in the Warm group, and 177 (9.8%) in the Hot group.
Compared with the Cold group, patients in the Cool group, Warm group, and Hot group, had a lower baseline NIHSS score (19 [13–28] vs 17 [13–23.8] vs 17 [13–22] vs 17 [12–21.5], P=0.032), lower onset to revascularization time (443 [305–645] vs 412.5 [300.5–630.8] vs 440 [308–678] vs 401[269–538], P=0.042), lower incidence of ischemic stroke (26.5% vs 16.3% vs 17.2 vs 23.2%, P=0.004), lower incidence of general anesthesia (40.9% vs 34.5% vs 31.8% vs 27.7%, P=0.038) and stroke causative mechanism (eg, large artery atherosclerosis 55.2% vs 44.6% vs 52.5% vs 53.7%, P=0.020), and a difference in patients with atrial fibrillation (28.7% vs 35.2% vs 29.7% vs 25.4%, P=0.024) and patients with IV thrombolysis (13.3% vs 15.2% vs 11.4% vs 20.3%, P=0.012), and a significant difference in occlusion sites (anterior circulation: 53.0% vs 63.5% vs 68.8 vs 71.2%, P < 0.001; posterior circulation: 47.0% vs 36.5% vs 31.2% vs 28.8%, P < 0.001). Other baseline characteristics did not differ significantly between the groups (P > 0.05) (Table 1).
Table 1.
Baseline Characteristics of the Study Population: Patients with Acute LVO
| Characteristics | No/Total No. (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Cold Group | Cool Group | Warm Group | Hot Group | χ2/z Value | P value | ||
| Number of patients | 1809 | 181 | 724 | 727 | 177 | |||
| Age, years, median (IQR) | 67(57–74) | 64(54–72.5) | 66(56–74) | 67(58–74) | 70(59–76) | 15.062 | 0.002 | |
| ≥67 | 912/1809(50.4) | 80/181(44.2) | 355/724(49.0) | 372/727(51.2) | 105/177(59.3) | 9.133 | 0.028 | |
| Male sex, n (%) | 1159/1809(64.1) | 125/181(69.1) | 472/724(65.2) | 449/727(61.8) | 113/177(63.8) | 4.043 | 0.257 | |
| Baseline NIHSS score, median (IQR) | 17(13–23) | 19(13–28) | 17(13–23.8) | 17(13–22) | 17(12–21.5) | 8.808 | 0.032 | |
| ≥17 | 987/1809(54.6) | 112/181(61.9) | 399/724(55.1) | 384/727(52.8) | 92/177(52.0) | 5.363 | 0.147 | |
| ASITN/SIR grade† | ||||||||
| 0–1 | 734/1808(40.6) | 86/181(47.5) | 301/724(41.6) | 277/727(38.1) | 70/176(39.8) | 9.1312 | 0.166 | |
| 2 | 660/1808(36.5) | 58/181(32.0) | 271/724(37.4) | 263/727(36.2) | 68/176(38.6) | |||
| 3–4 | 414/1808(22.9) | 37/181(20.4) | 152/724(21.0) | 187/727(25.7) | 38/176(21.6) | |||
| Baseline ASPECT score, median (IQR) ‡ | 8(7–9) | 8(6–9) | 8(7–9) | 8(7–9) | 8(7–9) | 4.497 | 0.213 | |
| ≥8 | 1000/1805(55.4) | 91/180(50.6) | 409/722(56.6) | 397/726(54.7) | 103/177(58.2) | 2.874 | 0.411 | |
| Blood pressure on admission, median (IQR), mmHg** | 147(130–163) | 146(130–169) | 145(130–162) | 148(131–163) | 145(130–161.8) | 1.906 | 0.592 | |
| Medical history, No. (%) | ||||||||
| Hypertension | 1103/1809(61.0) | 118/181(65.2) | 437/724(60.4) | 440/727(60.5) | 108/177 (61.0) | 1.532 | 0.675 | |
| Hyperlipidemia | 379/1809(21.0) | 40/181(22.1) | 152/724(21.0) | 149/727(20.5) | 38/177(21.5) | 0.265 | 0.966 | |
| Diabetes | 391/1809(21.6) | 33/181(18.2) | 159/724(22.0) | 164/727(22.6) | 35/177(19.8) | 2.010 | 0.570 | |
| Smoking | 507/1809(28.0) | 62/181(34.3) | 203/724(28.0) | 194/727(26.7) | 48/177(27.1) | 4.201 | 0.241 | |
| Ischemic stroke | 332/1809(18.4) | 48/181(26.5) | 118/724(16.3) | 125/727(17.2) | 41/177 (23.2) | 13.481 | 0.004 | |
| Cerebral hemorrhage | 27/1809(1.5) | 5/181(2.8) | 11/724(1.5) | 6/727(0.8) | 5/177 (2.8) | 5.913 | 0.116 | |
| TIA | 18/1809(1.0) | 3/181(1.7) | 5/724(0.7) | 10/727(1.4) | 0/177 (0.0) | 5.929 | 0.115 | |
| Coronary heart disease | 304/1809(16.8) | 22/181(12.2) | 127/724(17.5) | 125/727(16.9) | 30/177 (16.9) | 3.162 | 0.367 | |
| Valvular heart disease or infective endocarditis | 126/1809 (7.0) | 10/181(5.5) | 62/724(8.6) | 45/727(6.2) | 9/177 (5.1) | 5.074 | 0.166 | |
| Atrial fibrillation | 568/1809(31.4) | 52/181(28.7) | 255/724(35.2) | 216/727(29.7) | 45/177(25.4) | 9.404 | 0.024 | |
| Treatment profiles | ||||||||
| Onset to groin puncture, median (IQR), min | 324(215–535) | 332(222.5–527.5) | 320.5(218–535.7) | 335(215–570) | 296(195.5–443) | 7.574 | 0.056 | |
| Onset to revascularization, median (IQR), min | 428(302–640.3) | 443(305–645) | 412.5(300.5–630.8) | 440(308–678) | 401(269–538) | 8.183 | 0.042 | |
| Groin puncture to revascularization, median (IQR), min | 80(50–126) | 86(55–134) | 77(49–122) | 81(50–126) | 74(48–127.5) | 3.753 | 0.289 | |
| Stroke causative mechanism | ||||||||
| Large artery atherosclerosis | 900/1809(49.8) | 100/181(55.2) | 323/724(44.6) | 382/727(52.5) | 95/177(53.7) | 19.700 | 0.020 | |
| Cardioembolism | 707/1809(39.1) | 58/181(32.0) | 315/724(43.5) | 271/727(37.3) | 63/177(35.6) | |||
| Other | 90/1809(5.0) | 12/181(6.6) | 43/724(5.9) | 30/727(4.1) | 5/177(2.8) | |||
| Unknown | 112/1809(6.2) | 11/181(6.1) | 43/724(5.9) | 44/727(6.1) | 14/177(7.9) | |||
| General anesthesia, No. (%) * | 601/1801(33.4) | 74/181(40.9) | 248/719(34.5) | 230/724(31.8) | 49/177(27.7) | 8.413 | 0.038 | |
| IV Thrombolysis | 253/1809(14.0) | 24/181(13.3) | 110/724(15.2) | 83/727(11.4) | 36/177(20.3) | 10.884 | 0.012 | |
| Occlusion sites | ||||||||
| Anterior circulation | 1182/1809(65.3) | 96/181(53.0) | 460/724(63.5) | 500/727(68.8) | 126/177(71.2) | 19.596 | 0.000 | |
| Posterior circulation | 627/1809(34.7) | 85/181(47.0) | 264/724(36.5) | 227/727(31.2) | 51/177(28.8) | |||
| mTICI score‡ | ||||||||
| 0–2a | 230/1805(12.7) | 21/180(11.7) | 96/723(13.3) | 93/726(12.8) | 20/176(11.4) | 0.678 | 0.878 | |
| 2b or 3 | 1575/1805(87.3) | 159/180(88.3) | 627/723(86.7) | 633/726(87.2) | 156/176(88.6) | |||
Notes: *Missing data in 8 patient, †Missing data in 1 patients, ‡Missing data in 4 patients.
Abbreviations: IQR, interquartile range; NIHSS, National Institutes of Health Stroke Scale; ASPECTS, Alberta Stroke Program Early CT Score; mTICI, modified thrombolysis in cerebral infarction; IV, intravenous.
Primary Effectiveness Outcome
The median modified Rankin Scale (mRS) score at 90 days was 4 (IQR, 1.5 to 6) in the Cold group, 3 IQR, 1 to 6) in the Cool group, 3 (IQR, 1 to 6) in the Warm group, and 3 (IQR,1 to 5) in the Hot group. The statistical analysis showed a significant difference in outcomes between the groups (P=0.050). After adjusting for confounding factors, the common odds ratio (OR) for improved functional outcome with mean temperature onset to groin puncture was 1.006 (95% confidence interval [CI]: 0.997–1.016, P=0.179), indicating no significant association. However, when comparing the Warm group to the Cold group, the common odds ratio (OR) for improved functional outcome was 1.386 (95% confidence interval [CI]: 1.024–1.878, P=0.035) after adjusting for confounding factors. This suggests that patients in the Warm group had a higher likelihood of improved functional outcome compared to those in the Cold group (Figure 2, Tables 2 and 3).
Figure 2.
Distribution of the modified Rankin scale score at 90 days of Cold, Cool, Warm, and Hot groups. The distribution of the modified Rankin scale score of primary outcomes in four groups derived on the temperature for all patients treated with endovascular treatment.
Table 2.
Primary Outcomes and Secondary Outcomes
| Characteristics | Unadjusted Outcome Variable Value (95% CI) | P value | Adjusted Value (95% CI) | P value |
|---|---|---|---|---|
| Primary Efficacy Outcomes | ||||
| Modified Rankin Scale score at 90d, median (IQR) | ||||
| Mean temperature Onset to groin puncture (°C) | 1.013(1.004–1.022) | 0.004 | 1.006(0.997–1.016) | 0.179 |
| Cold group (≤6.47) | Reference | NA | Reference | NA |
| Cool group (6.48–19.78) | 1.349(1.009–1.804) | 0.044 | 1.311(0.968–1.776) | 0.080 |
| Warm group (19.79–29.83) | 1.499(1.121–2.003) | 0.006 | 1.386(1.024–1.878) | 0.035 |
| Hot group (≥29.84) | 1.472(1.022–2.119) | 0.038 | 1.254(0.856–1.838) | 0.246 |
| Secondary Efficacy Outcomes | ||||
| mRS 90d, 0–3 | ||||
| Mean temperature Onset to groin puncture (°C) | 1.011(1.001–1.021) | 0.038 | 1.005(0.993–1.018) | 0.407 |
| Cold group (≤6.47) | Reference | NA | Reference | NA |
| Cool group (6.48–19.78) | 1.290(0.930–1.790) | 0.127 | 1.234(0.833–1.828) | 0.293 |
| Warm group (19.79–29.83) | 1.510(1.089–2.095) | 0.014 | 1.474(0.994–2.185) | 0.053 |
| Hot group (≥29.84) | 1.367(0.902–2.072) | 0.141 | 1.189(0.725–1.947) | 0.493 |
| mRS 90d, 0–2 | ||||
| Mean temperature Onset to groin puncture (°C) | 1.016(1.006–1.027) | 0.002 | 1.016(1.004–1.029) | 0.009 |
| Cold group (≤6.47) | Reference | NA | Reference | NA |
| Cool group (6.48–19.78) | 1.366(0.968–1.928) | 0.076 | 1.322(0.889–1.965) | 0.168 |
| Warm group (19.79–29.83) | 1.599(1.134–2.254) | 0.007 | 1.646(1.107–2.448) | 0.014 |
| Hot group (≥29.84) | 1.520(0.988–2.339) | 0.057 | 1.460(0.890–2.394) | 0.134 |
| mRS 90d, 0–1 | ||||
| Mean temperature Onset to groin puncture (°C) | 1.007(0.996–1.018) | 0.233 | 1.005(0.993–1.018) | 0.406 |
| Cold group (≤6.47) | Reference | NA | Reference | NA |
| Cool group (6.48–19.78) | 1.285(0.885–1.866) | 0.187 | 1.232(0.810–1.875) | 0.330 |
| Warm group (19.79–29.83) | 1.286(0.886–1.867) | 0.186 | 1.267(0.832–1.929) | 0.270 |
| Hot group (≥29.84) | 1.157(0.722–1.853) | 0.544 | 1.087(0.640–1.845) | 0.757 |
| Safety outcomes | ||||
| Symptomatic intracranial hemorrhage, No. (%) | ||||
| Mean temperature Onset to groin puncture (°C) | 0.995(0.976–1.014) | 0.596 | 0.997(0.977–1.018) | 0.802 |
| Cold group (≤6.47) | Reference | NA | Reference | NA |
| Cool group (6.48–19.78) | 1.062(0.579–1.940) | 0.847 | 0.960(0.514–1.794) | 0.898 |
| Warm group (19.79–29.83) | 0.922(0.499–1.703) | 0.795 | 0.877(0.466–1.651) | 0.684 |
| Hot group (≥29.84) | 0.719(0.310–1.664) | 0.440 | 0.726(0.307–1.715) | 0.465 |
| Complications after cerebral infarction, No. (%) | ||||
| Pneumonia | ||||
| Mean temperature Onset to groin puncture (°C) | 0.985(0.975–0.996) | 0.005 | 0.992(0.981–1.003) | 0.170 |
| Cold group (≤6.47) | Reference | NA | Reference | NA |
| Cool group (6.48–19.78) | 1.092(0.781–1.527) | 0.606 | 1.271(0.884–1.828) | 0.196 |
| Warm group (19.79–29.83) | 0.853(0.611–1.191) | 0.350 | 1.016(0.707–1.459) | 0.933 |
| Hot group (≥29.84) | 0.765(0.502–1.165) | 0.211 | 0.952(0.604–1.503) | 0.834 |
| Mortality at 90 days, No. (%)* | ||||
| Mean temperature Onset to groin puncture (°C) | 0.984(0.973–0.995) | 0.004 | 0.992(0.979–1.005) | 0.208 |
| Cold group (≤6.47) | Reference | NA | Reference | NA |
| Cool group (6.48–19.78) | 0.727(0.515–1.026) | 0.070 | 0.717(0.478–1.076) | 0.109 |
| Warm group (19.79–29.83) | 0.633(0.447–0.896) | 0.010 | 0.667(0.444–1.004) | 0.052 |
| Hot group (≥29.84) | 0.569(0.359–0.902) | 0.016 | 0.653(0.381–1.118) | 0.120 |
Note: *All cause death.
Abbreviations: IQR, interquartile range; CI, Confidence Interval; mRS, Modified Rankin Scale score.
Table 3.
Efficacy Outcomes and Safety Outcomes
| Characteristics | No/Total No. (%) | ||||||
|---|---|---|---|---|---|---|---|
| All (n=1809) | Cold Group | Cool Group | Warm Group | Hot Group | χ2/z Value | P value | |
| Primary Efficacy Outcomes | |||||||
| Modified Rankin Scale score at 90d, median (IQR) | 3(1–6) | 4(1.5–6) | 3(1–6) | 3(1–6) | 3(1–5) | 7.797 | 0.050 |
| Secondary Efficacy Outcomes | |||||||
| Modified Rankin Scale score at 90d, No. (%) | |||||||
| 0–3, No. (%) | 944/1809(52.2) | 81/181(44.8) | 370/724(51.1) | 400/727(55.0) | 93/177(52.5) | 6.699 | 0.082 |
| 0–2, No. (%) | 739/1809(40.9) | 59/181(32.6) | 288/724(39.8) | 317/727(43.6) | 75/177(43.4) | 7.898 | 0.048 |
| 0–1, No. (%) | 527/1809(29.1) | 45/181(24.9) | 216/724(29.8) | 217/727(29.8) | 49/177(27.7) | 2.132 | 0.545 |
| Safety outcomes | |||||||
| Symptomatic intracranial hemorrhage, No. (%)† | 135/1804(7.5) | 14/181(7.7) | 59/722(8.2) | 52/725(7.5) | 10/176(5.7) | 1.437 | 0.697 |
| Complications after cerebral infarction, No. (%) | |||||||
| Cerebral hernia | 131/1809(7.2) | 18/181(9.9) | 43/724(5.9) | 57/727(7.8) | 13/177(7.3) | 4.188 | 0.242 |
| Decompressive craniectomy | 50/1809(2.8) | 4/181(2.2) | 21/724(2.9) | 19/727(2.6) | 6/177(3.4) | 0.576 | 0.902 |
| Pneumonia | 1085/1809(60.0) | 111/181(61.3) | 459/724(63.4) | 418/727(57.5) | 97/177(54.8) | 7.504 | 0.057 |
| Respiratory failure | 426/1809(23.5) | 46/181(25.4) | 181/724(25.0) | 165/727(22.7) | 34/177(19.2) | 3.342 | 0.342 |
| Circulatory failure | 267/1809(14.8) | 24/181(13.3) | 113/724(15.6) | 105/727(14.4) | 25/177(14.1) | 0.852 | 0.837 |
| Venous thrombosis | 169/1809(9.3) | 19/181(10.5) | 73/724(10.1) | 62/727(8.5) | 15/177(8.5) | 1.480 | 0.687 |
| Death in hospital | 92/1809(5.1) | 8/181(4.4) | 40/724(5.5) | 35/727(4.8) | 9/177(5.1) | 0.566 | 0.904 |
| Mortality at 90 days, No. (%)* | 499/1809(27.6) | 64/181(35.4) | 206/724(28.5) | 187/727(25.7) | 42/177(23.7) | 8.330 | 0.040 |
Notes: *All cause death; †Missing data in 5 patients.
Abbreviations: IQR, interquartile range; CI, Confidence Interval.
Secondary Effectiveness Outcome
The mRS (0–2 vs.3–6) at 90 days, the common odds ratio (OR) for improved functional outcome with Mean temperature onset to groin puncture was 1.016 (95% confidence interval [CI]: 1.004–1.029, P=0.009) after adjustment for confounding factors; At 90 days, 317/727 (43.6%) patients in the Warm group compared with 59/181 (32.6%) patients in the Cold group had a favorable functional outcome (adjusted OR: 1.646, 95% CI: 1.107–2.448, P=0.014; Table 2 and Table 3).
Safety Outcomes
Overall, sICH occurred in 135/1809 (missing data in 5 patients) (7.5%) patients: 7.7% in the Cold group, 8.2% in the Cool group, 7.5% in the Warm group, and 5.7% in the Hot group, which was no significantly differences of sICH occurred between groups (eg, adjusted OR: 0.997, P=0.802, Table 2), pneumonia in the Cold group was 61.3%, in the Cool group was 63.4%, in the Warm group was 57.5%, in the Hot group was 54.8%, which was no significantly differences of pneumonia between groups (eg, adjusted OR: 0.992, P=0.170, Table 2), and mortality in the Cold group was 35.4%, in the Cool group was 28.5%, in the Warm group was 25.7%, in the Hot group was 23.7%, which was no significantly differences of mortality rate between groups (eg, adjusted OR: 0.992, P=0.208, Table 3).
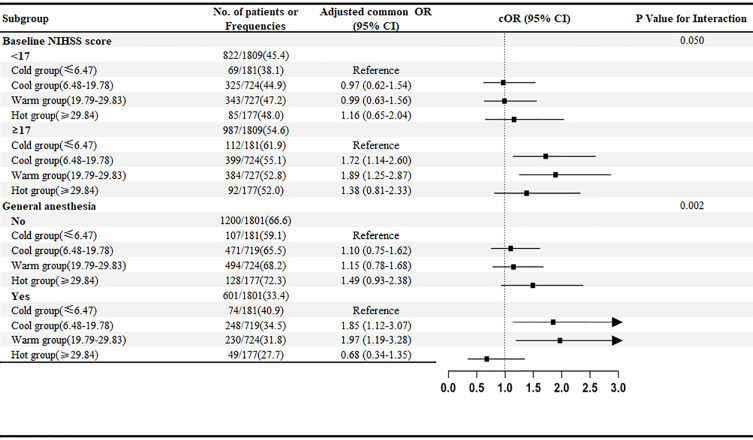
Subgroup Analysis
As shown in Figure 3 and Supplementary Figures S1–S5, the results of subgroup analyses suggested a potential heterogeneity in the effect of temperature on functional outcomes among patients with different severity of neurological deficits (P for interaction = 0.050) and those with different anesthesia strategies (P for interaction < 0.01).
Figure 3.
The forest plot displays effect variation across subgroups for the adjusted common odds ratio of less disability at 90 days as compared with the Cold group. The forest plot displays effect variation across 11 subgroups for the adjusted common odds ratio of less disability at 90 days. A lower modified Rankin Scale (mRS) score indicates less disability. The thresholds for age, baseline National Institutes of Health Stroke Scale score (NIHSS), and onset to recanalization time were chosen at the median. Adjusted odds ratio (95% CI) > 3 indicated by arrows.
In patients with more severe neurologic deficits, compared with the Cold group, the Cool group, and Warm group were statistically associated with better functional prognosis (1.719 (95% confidence interval [CI]: 1.135–2.601, P=0.010) and 1.892 (95% confidence interval [CI]: 1.249–2.866, P=0.003), while the Hot Group did not show a statistically significant association with a better functional prognosis (1.376 (95% confidence interval [CI]: 0.811–2.334, P=0.237). In contrast, in patients with NIHSS scores <17, the association of temperature with functional prognosis was not observed. A stronger association between temperature and functional outcome can also be observed in patients with general anesthesia than in patients without general anesthesia.
However, statistically significant heterogeneity was not found among patients with different age, sex, intravenous thrombolysis, occlusion site, onset to recanalization time, previous ischemic stroke, atrial fibrillation, and recanalization.
Discussion
This study represents the inaugural research endeavor to investigate ambient temperature in Chinese patients with AIS due to LVO undergoing EVT. Our observations indicated that the mean temperature from stroke onset to groin puncture was associated with a decrease in mortality and enhancements in functional outcomes at 90 days. To clarify the effect of mean temperature from stroke onset to groin puncture on the nerve function of patients with AIS, we utilized a national comprehensive database from three studies and obtained mean temperature from onset to groin puncture. Our results show that Warm ambient temperature is strongly associated with good functional prognosis compared with Cold ambient temperature, whereas Cool and Hot ambient temperatures have no such association.
Many epidemiologic studies have stated an affiliation between ambient temperature and stroke, even though the actual findings were inconsistent. A case-crossover found that short-term decrement in ambient temperature heightened risk of ischemic stroke.3 Similar findings on low temperature and greater stroke incidence had been additionally mentioned in different studies.6,17,18 Another aspect, other registrants believe that high ambient temperature was linked to higher stroke onset and mortality.19–22 Multiple studies have indicated that they did not find a significant connection between outdoor temperature and ischemic stroke.14–16,23 Contrary to the above views, the “ISOTHURM” project observed that urban populations are affected by rising mortality rates due to both high and low temperatures.24 Using records from 8 massive cities in China came to a similar conclusion: temperature and stroke mortality have been frequently nonlinear—both low and excessive temperatures had been linked to greater stroke deaths.13 Our study revealed that patients in the Warm group exhibited higher rates of 90-day favorable outcomes and functional independence, as well as a lower mortality rate compared to patients in the Cold group. The precise reasons for these variations are difficult to disentangle, but may contain variations in fitness care systems, learning populations, geography, duration of the study, meteorological characteristics, research designs, and analytical strategies.
Several mechanisms may explain our findings. When exposed to cold, the sympathetic nervous system is triggered and releases substances such as catecholamines that aid in generating heat, sympathetic-mediated peripheral vasoconstriction and marked increase in blood pressure caused by a decrease in skin temperature, the renin-angiotensin-aldosterone system is known to play a crucial in cold-induced blood pressure elevation, and its role may be related to the inhibition of nitric oxide synthesis, and urinary sodium excretion may also be related to temperature-induced changes in blood pressure. However, high temperature is associated with deterioration of endothelial function, on the other hand, the expansion of blood vessels and massive sweating of the skin under high temperature may lead to low blood volume and loss of sodium salt, which may cause a decrease in blood pressure.25,26 Ambient temperature also influences platelet count, heart rate, platelet viscosity, peripheral vasoconstriction function, plasma fibrinogen, cholesterol, and inflammation markers.27,28 Based on this study, while individuals cannot entirely evade exposure to cold temperatures, those with cerebrovascular disease and an elevated risk of stroke should minimize prolonged exposure to cold outdoor conditions or take appropriate measures to stay warm.
Our discovery was groundbreaking as it centered on a population with acute ischemic stroke (AIS) caused by large vessel occlusion (LVO) undergoing endovascular therapy (EVT). The regions studied cover a wide range of geographical areas, different seasons, and large target populations. This approach provided adequate statistical power to explore the association between temperature and stroke outcomes.
We should acknowledge several limitations of our study. First, being an observational study, bias from patient selection was unavoidable. While confounding factors can be adjusted through multivariate logistic analysis, there are still systematic differences among subgroups. Second, we utilized the average outdoor temperature from stroke onset to groin puncture in the analysis, which may have underestimated the impact of exposure to extreme temperatures. Third, this study only included patients with large vessel occlusion who underwent endovascular treatment. However, as there are several pathophysiology, prognosis, and clinical features between large vessel occlusion and lacunar strokes, it is important to investigate the effect of ambient temperature in a more broad population. Fourth, it would be useful to mention the causes of death (neurological and non-neurological) in the study sample. Fifth, our studies have focused on mainland China, and there is a lack of information on other species in the broader region of Asia and beyond. Finally, further extensive research is necessary to enhance the comprehension of the moderating effects of humidity, air pressure, air pollutants, latitude and longitude factors, etc.
Conclusion
In summary, warm temperatures have been shown to enhance functional outcomes at 90 days and decrease the likelihood of death in LVO patients undergoing EVT compared to cold temperatures. However, additional randomized clinical trials are necessary to validate the findings of this study.
Acknowledgments
We express our gratitude to all the co-investigators of RESCUE BT, DEVT, and BASILAR for their unwavering dedication to the study.
Disclosure
The authors declare no conflicts of interest in this study.
References
- 1.Xiong Y, Wakhloo AK, Fisher M. Advances in Acute Ischemic Stroke Therapy. Circ Res. 2022;130(8):1230–1251. doi: 10.1161/CIRCRESAHA.121.319948 [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Stark BA, Johnson CO; Collaborators GBDS. Global, regional, and national burden of stroke and its risk factors, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet Neurol. 2021;20(10):795–820. doi: 10.1016/S1474-4422(21)00252-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mostofsky E, Wilker EH, Schwartz J, et al. Short-term changes in ambient temperature and risk of ischemic stroke. Cerebrovasc Dis Extra. 2014;4(1):9–18. doi: 10.1159/000357352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rakers F, Schiffner R, Rupprecht S, et al. Rapid weather changes are associated with increased ischemic stroke risk: a case-crossover study. Eur J Epidemiol. 2016;31(2):137–146. doi: 10.1007/s10654-015-0060-3 [DOI] [PubMed] [Google Scholar]
- 5.Feigin VL, Nikitin YP, Bots ML, Vinogradova TE, Grobbee DE. A population-based study of the associations of stroke occurrence with weather parameters in Siberia, Russia (1982–1992). Eur J Neurol. 2000;7(2):171–178. doi: 10.1046/j.1468-1331.2000.00016.x [DOI] [PubMed] [Google Scholar]
- 6.Hong YC, Rha JH, Lee JT, Ha EH, Kwon HJ, Kim H. Ischemic stroke associated with decrease in temperature. Epidemiology. 2003;14(4):473–478. doi: 10.1097/01.ede.0000078420.82023.e3 [DOI] [PubMed] [Google Scholar]
- 7.Chang CL, Shipley M, Marmot M, Poulter N. Lower ambient temperature was associated with an increased risk of hospitalization for stroke and acute myocardial infarction in young women. J Clin Epidemiol. 2004;57(7):749–757. doi: 10.1016/j.jclinepi.2003.10.016 [DOI] [PubMed] [Google Scholar]
- 8.Ohshige K, Hori Y, Tochikubo O, Sugiyama M. Influence of weather on emergency transport events coded as stroke: population-based study in Japan. Int J Biometeorol. 2006;50(5):305–311. doi: 10.1007/s00484-005-0018-3 [DOI] [PubMed] [Google Scholar]
- 9.Ebi KL, Exuzides KA, Lau E, Kelsh M, Barnston A. Weather changes associated with hospitalizations for cardiovascular diseases and stroke in California, 1983–1998. Int J Biometeorol. 2004;49(1):48–58. doi: 10.1007/s00484-004-0207-5 [DOI] [PubMed] [Google Scholar]
- 10.Han MH, Yi HJ, Kim YS, Kim YS. Effect of seasonal and monthly variation in weather and air pollution factors on stroke incidence in Seoul, Korea. Stroke. 2015;46(4):927–935. doi: 10.1161/STROKEAHA.114.007950 [DOI] [PubMed] [Google Scholar]
- 11.Vered S, Paz S, Negev M, Tanne D, Zucker I, Weinstein G. High ambient temperature in summer and risk of stroke or transient ischemic attack: a national study in Israel. Environ Res. 2020;187:109678. doi: 10.1016/j.envres.2020.109678 [DOI] [PubMed] [Google Scholar]
- 12.Lian H, Ruan Y, Liang R, Liu X, Fan Z. Short-term effect of ambient temperature and the risk of stroke: a systematic review and meta-analysis. Int J Environ Res Public Health. 2015;12(8):9068–9088. doi: 10.3390/ijerph120809068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen R, Wang C, Meng X, et al. Both low and high temperature may increase the risk of stroke mortality. Neurology. 2013;81(12):1064–1070. doi: 10.1212/WNL.0b013e3182a4a43c [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cevik Y, Dogan NO, Das M, Ahmedali A, Kul S, Bayram H. The association between weather conditions and stroke admissions in Turkey. Int J Biometeorol. 2015;59(7):899–905. doi: 10.1007/s00484-014-0890-9 [DOI] [PubMed] [Google Scholar]
- 15.Zorrilla-Vaca A, Healy RJ, Silva-Medina MM. Revealing the association between cerebrovascular accidents and ambient temperature: a meta-analysis. Int J Biometeorol. 2017;61(5):821–832. doi: 10.1007/s00484-016-1260-6 [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto M, Ishikawa S, Kajii E. Cumulative effects of weather on stroke incidence: a multi-community cohort study in Japan. J Epidemiol. 2010;20(2):136–142. doi: 10.2188/jea.JE20090103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magalhaes R, Silva MC, Correia M, Bailey T. Are stroke occurrence and outcome related to weather parameters? Results from a population-based study in northern Portugal. Cerebrovasc Dis. 2011;32(6):542–551. doi: 10.1159/000331473 [DOI] [PubMed] [Google Scholar]
- 18.Goggins WB, Woo J, Ho S, Chan EY, Chau PH. Weather, season, and daily stroke admissions in Hong Kong. Int J Biometeorol. 2012;56(5):865–872. doi: 10.1007/s00484-011-0491-9 [DOI] [PubMed] [Google Scholar]
- 19.Basu R. High ambient temperature and mortality: a review of epidemiologic studies from 2001 to 2008. Environ Health. 2009;8(1):40. doi: 10.1186/1476-069X-8-40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giua A, Abbas MA, Murgia N, Corea F. Climate and stroke: a controversial association. Int J Biometeorol. 2010;54(1):1–3. doi: 10.1007/s00484-009-0253-0 [DOI] [PubMed] [Google Scholar]
- 21.Low RB, Bielory L, Qureshi AI, Dunn V, Stuhlmiller DF, Dickey DA. The relation of stroke admissions to recent weather, airborne allergens, air pollution, seasons, upper respiratory infections, and asthma incidence, September 11, 2001, and day of the week. Stroke. 2006;37(4):951–957. doi: 10.1161/01.STR.0000214681.94680.66 [DOI] [PubMed] [Google Scholar]
- 22.Basu R, Samet JM. Relation between elevated ambient temperature and mortality: a review of the epidemiologic evidence. Epidemiol Rev. 2002;24(2):190–202. doi: 10.1093/epirev/mxf007 [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Cao Y, Hong D, et al. Ambient temperature and stroke occurrence: a systematic review and meta-analysis. Int J Environ Res Public Health. 2016;13:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McMichael AJ, Wilkinson P, Kovats RS, et al. International study of temperature, heat and urban mortality: the ‘ISOTHURM’ project. Int J Epidemiol. 2008;37(5):1121–1131. doi: 10.1093/ije/dyn086 [DOI] [PubMed] [Google Scholar]
- 25.Jehn M, Appel LJ, Sacks FM, Miller ER; Group DCR. The effect of ambient temperature and barometric pressure on ambulatory blood pressure variability. Am J Hypertens. 2002;15(11):941–945. doi: 10.1016/S0895-7061(02)02999-0 [DOI] [PubMed] [Google Scholar]
- 26.Stergiou GS, Palatini P, Modesti PA, et al. Seasonal variation in blood pressure: evidence, consensus and recommendations for clinical practice. Consensus statement by the European Society of Hypertension working group on blood pressure monitoring and cardiovascular variability. J Hypertens. 2020;38(7):1235–1243. doi: 10.1097/HJH.0000000000002341 [DOI] [PubMed] [Google Scholar]
- 27.Stout RW, Crawford V. Seasonal variations in fibrinogen concentrations among elderly people. Lancet. 1991;338(8758):9–13. doi: 10.1016/0140-6736(91)90004-9 [DOI] [PubMed] [Google Scholar]
- 28.Nawrot TS, Staessen JA, Fagard RH, Van Bortel LM, Struijker-Boudier HA. Endothelial function and outdoor temperature. Eur J Epidemiol. 2005;20(5):407–410. doi: 10.1007/s10654-005-1068-x [DOI] [PubMed] [Google Scholar]