Abstract
OBJECTIVE:
To measure the protein concentration and biological activity of HIV-1 Tat in cerebrospinal fluid (CSF) of individuals on suppressive antiretroviral therapy (ART).
DESIGN:
CSF was collected from 68 HIV-positive individuals on ART with plasma viral load (VL) <40 copies/mL, and from 25 HIV-negative healthy controls. Duration of HIV infection ranged from 4 years to >30 years.
METHODS:
Tat levels in CSF were evaluated by an enzyme-linked immunosorbent assay (ELISA). Tat protein and viral RNA were quantified from exosomes isolated from CSF, followed by western blot or quantitative reverse transcription PCR, respectively. Functional activity of Tat was assessed using an LTR transactivation assay.
RESULTS:
Tat protein was detected in 36.8% of CSF samples from HIV-positive patients. CSF Tat concentration increased in 4 out of 5 individuals after initiation of therapy, indicating that Tat was not inhibited by ART. Similarly, exosomes from 34.4% of CSF samples were strongly positive for Tat protein and/or TAR RNA. Exosomal Tat retained transactivation activity in a CEM-LTR reporter assay in 66.7% of samples assayed, which indicates that over half of the Tat present in CSF is functional. Presence of Tat in CSF was highly associated with previous abuse of psychostimulants (cocaine or amphetamines; p=0.01) and worse performance in the psychomotor speed (p=0.04) and information processing (p=0.02) cognitive domains.
CONCLUSIONS:
Tat and TAR are produced in the CNS despite adequate ART and are packaged into CSF exosomes. Tat remains biologically active within this compartment. These studies suggest that Tat may be a quantifiable marker of the viral reservoir and highlight a need for new therapies that directly inhibit Tat.
Keywords: HIV-1 reservoir, HIV-1 associated neurocognitive disorder, drug abuse, comorbidity
INTRODUCTION:
Advances in HIV research have vastly extended life expectancy and reduced HIV transmission rates within the HIV infected community. Nonetheless, HIV remains a chronic infection with long-term health care concerns including immunological senescence and dysfunction and HIV-associated neurocognitive disorders (HAND) [1]. The incidence of severe impairment such as HIV-associated dementia has decreased since the introduction of antiretroviral therapy (ART), but milder forms of HAND still affect 30–50% of the those living with HIV [2, 3].
The HIV Trans-Activator of Transcription (Tat) is a key regulatory protein that impacts several aspects of viral replication and HIV disease progression. Tat is one of the first viral proteins to be translated after integration and greatly enhances transcriptional activity of the HIV promoter [4–6]. Tat functions as a “molecular switch” that regulates the cycle of active transcription versus latency [7]. Furthermore, Tat can be released from HIV-infected cells [8] and can modulate cell function in an autocrine, paracrine, or endocrine manner. Tat induces the production of reactive oxygen species (ROS) [9], cytokines, and chemokines (CCL2, TNFα, IL-1β, IL-6, IL-17) [10–15] that contribute to chronic inflammation. Tat also directly contributes to neuronal injury via interaction with the NMDA receptor [16–18] and dysregulation of astrocytic glutamate uptake and release [17, 19]. Thus, the detection of Tat in the CNS may be indicative of ongoing neuro-glial dysfunction.
Exosomes are extracellular vesicles 30–150 nm in diameter which are characterized by the presence of several proteins on their surface membrane, including the tetraspanins CD63, CD9, and CD81 [20–22]. Once thought to carry cellular waste out of cells, it is now known that exosomes shuttle cargo (including proteins and RNA) between cells [20, 22]. However, this cell-to-cell communication pathway can be hijacked by viruses, including HIV [23–25]. HIV-infected cells can release exosomes containing HIV RNA, specifically trans-activation response element (TAR), activate the NF-κB pathway through TLR3 activation and increase susceptibility of recipient cells to infection [24, 25]. Furthermore, exosomes from HIV-infected cells have been shown to contain HIV proteins, including Nef and Tat [26, 27].
Several classes of anti-HIV drugs are available that act at various stages of the HIV life cycle. These include HIV entry inhibitors, reverse transcriptase inhibitors, protease inhibitors, and integrase inhibitors which may impact the formation of the viral reservoir. However, recent studies show that HIV enters the CNS early in infection [28–30]; thus, the viral reservoir gets established before antiretroviral therapy is initiated. Once the virus is integrated, HIV protease inhibitors are the only class of drugs that prevent the formation of replicating viral particles. Furthermore, while protease inhibitors prevent the cleavage of the gag-pol polyprotein, they have no effect on the production of early viral proteins such as Tat [15]. Thus, Tat protein levels may indicate the presence and potentially the size of the viral reservoir. This is of particular importance, as there are currently no reliable methods for measuring the viral reservoir in the brain. It is also unknown if prolonged ART can alter the size of the viral reservoir and if it can completely control viral replication and production of viral products in the brain.
METHODS:
Cells and infection studies:
Peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors at the NIH Blood Bank (Bethesda, MD). Monocytes were isolated by adherence and differentiated into macrophages (MDM) by culture in RPMI 1640 (Thermo Fisher; Waltham, MA) supplemented with 10% (v/v) fetal bovine serum and 1% (v/v) antibiotic-antimycotic for ≥ 7 days. MDM were infected with HIVSF162 (>1 ng p24 per million cells) using polybrene (5 μg/ mL) (Sigma-Aldrich; St. Louis, MO) for four hours, then cells were washed with PBS and medium was replaced with complete RPMI containing dimethyl sulfoxide (DMSO) or darunavir (1 μM in DMSO). Darunavir was obtained through the NIH AIDS Reagent Program (cat #11447), Division of AIDS, NIAID, NIH from Tibotec, Inc. Supernatants and cells were collected after seven days for product enhanced reverse transcriptase (PERT) assay [31] or Tat ELISA, respectively.
Transfection:
HIVSF162 virus stock was generated by transfection of HEK293T/17 (ATCC; Manassas, VA) with pSF162 plasmid using Lipofectamine 3000 (Thermo Fisher). pSF162 was obtained through the NIH AIDS Reagent Program (cat # 2569; discontinued, now available as cat # 2751), Division of AIDS, NIAID, NIH, from Dr. Jay Levy. Virus-containing supernatants were collected after 48 hours and filtered using a 0.4 μM membrane.
Patient samples:
CSF was collected from 68 HIV-infected individuals on ART (<40 copies/ mL in plasma) and 25 HIV-negative controls at the National Institutes of Health (NIH) Clinical Center in Bethesda, MD (NIH cohort; Protocol 13-N-0149). All participants in the NIH cohort were seen for research only and there were no clinical indications for CSF collection. CSF was also collected once from five HIV-positive individuals prior to ART initiation and once at a follow-up appointment after 2–5 months of continuous ART (UCSD cohort). Patient demographics for the NIH cohort are included in Fig. 4. The protocol was reviewed and approved by the Institutional Review Board (IRB) at both institutions. Informed consent was obtained from all individuals. All samples were centrifuged (3000 rpm, 10 minutes) and cell-free CSF was aliquoted in single-use vials and stored at −80°C.
Figure 4. Presence of Tat in CSF is associated with a history of drug abuse.
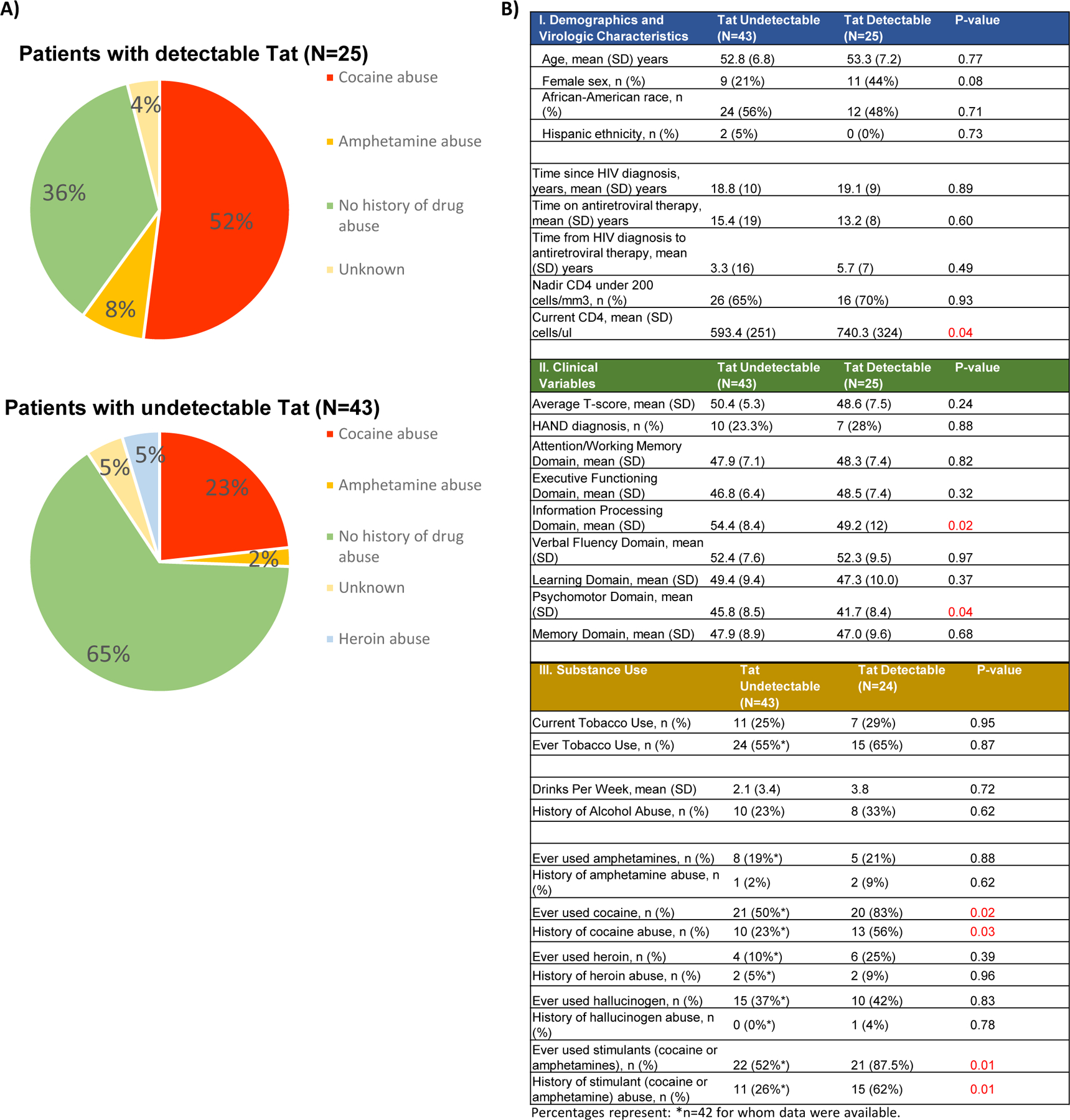
(A) Charts comparing percent of patients in the NIH cohort with Tat-positive (top) or Tat-negative (bottom) CSF who have a known history of drug abuse. Data on Illicit drug use was captured by a patient questionnaire asking each participant if he or she has ever used a specific substance. “Abuse of illicit drugs” includes patients who responded “yes” to questions regarding past drug use that affected their work or life but were negative for illegal substances by urine screening at their first visit. B) Summary of patient demographics for the NIH cohort (n=68), including sub-categories for patients with (n=25) or without (n=43) detectable CSF Tat. Significant differences between Tat-positive and Tat-negative individuals were determined by Chi-square analysis using Yate’s continuity correction, as appropriate for the sample size.
ELISA:
Tat ELISA was performed as previously described, with minor modifications [32]. Mouse anti-Tat antibody (Biolegend; San Diego, CA; Cat. # 919001) was used as capture antibody; the detection antibody was biotinylated rabbit anti-Tat (Abcam; Cambridge, MA, Cat. # ab43015). Recombinant clade B Tat protein (rTat; from Dr. Joseph Steiner) was used to generate a standard curve for quantitation of Tat levels in CSF. The limit of detection for this assay is 200 pg/ mL as determined by the mean O.D. obtained from HIV-negative CSF samples for each plate run + 2 standard deviations. Limit of detection was determined for each plate run and was consistent across plates. 2–5 CSF samples from HIV-negative controls were included in each run and all CSF was stored as single-use, never-thawed aliquots to ensure equal protein quality. Intra- and inter-plate coefficients of variation (%CV) were 5.75% and 8.45%, respectively, as indicated in supplementary Figure 1a-b. To confirm that the signals obtained from CSF samples increase or decrease uniformly with the dilution factor of the sample, 2-fold serial dilutions were performed on a known Tat-positive sample; as expected, calculated values for Tat protein decreased in a linear fashion with increasing dilution of the CSF (r2=0.966) (supplementary Figure 1c).
Isolation of exosomes from cerebrospinal fluid:
CSF (400 μL) was mixed 1:1 with PBS and treated with 20 μL of a 30% slurry of Nanotrap® (NT) particles (Ceres Nanosciences, Inc.; Manassas, VA) consisting of two NTs, NT82 particles (#CN2010) and NT80 (#CN1030), which have been previously shown to enrich for exosomes [25, 33]. Samples were rotated at 4°C for four days before use in downstream assays.
Quantification of HIV TAR RNA:
Exosomes were enriched using NT80/82 as described above. RNA was isolated from exosome-bound NT80/82 using Trizol (Thermo Fisher) and converted to cDNA using the GoScript kit (Promega; Madison, WI) with a TAR Reverse primer (5’-CAACAGACGGGCACACACTAC-3’, Tm=58°C). Reverse transcription-quantitative polymerase chain reaction (RT-qPCR) was performed using IQ Supermix (Bio-Rad; Hercules, CA) with primers TAR-Reverse and TAR-Forward (5’-GGTCTCTCTGGTTAGACCAGATCTG-3’, Tm=60°C). Serial dilutions of 8E5 cell DNA were used to generate a standard curve to quantitate TAR RNA. Real-time PCR reactions were carried out using the Bio-Rad CFX96 System. 8E5 cells were obtained through the NIH AIDS reagent program (cat. # 95), Division of AIDS, NIAID, NIH from Dr. Thomas Folks [34–36].
Western blot analysis of exosome proteins:
Exosomes were enriched as described above using NT80/82. 15 µL of each pellet was resuspended in Laemmli buffer and run on a 4–20% Tris/glycine gel (Thermo Fisher), then transferred onto Immobilon PVDF membranes (Millipore; Burlington, MA) overnight. Membranes were blocked in 5% milk in PBS-T (0.1% Tween-20) for two hours at 4°C, then incubated overnight at 4°C in PBS-T with primary antibody: anti-Tat (NIH AIDS reagent program; #705, Lot #100167 from Dr. Bryan R. Cullen [37]), anti-IgG (Santa Cruz, Dallas, TX; sc-66931), anti-CD63 (System Biosciences, Palo Alto, CA; EXOAB-CD63A-1), or anti-Actin (Abcam; ab49900). Membranes were incubated with HRP-conjugated secondary and visualized using Clarity Western ECL Substrate (Bio-Rad) and the ChemiDoc Touch system (Bio-Rad).
Tat transactivation assay:
CEM-GFP cells (cat. # 3655) were obtained through the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH from Dr. Jacques Corbeil [38]. Exosomes were enriched from 250 µL of CSF from six Tat-positive patients and two HIV-negative controls, as described above; 106 CEM-GFP cells were incubated with exosomes for 72 hours, then cells were washed in PBS and analyzed using the FACSCalibur (BD Biosciences; San Jose, CA); events were gated using CellQuest Pro using width and FL1 parameters (BD Biosciences). Untreated CEM-GFP cells and unbound NT80/82 treated CEM-GFP cells were used as negative controls and HIV virus (89.6; MOI: 1.0) was a positive control.
Substance Use History and Neurocognitive Function:
All participants in the NIH cohort completed questionnaires that included inventories of tobacco, alcohol and illicit drug use. Alcohol and drug abuse were defined as positive responses to questions asking about the frequency of substance use in the past and whether the substance use affected life or work. Additionally, all participants completed a 3–4 hour neuropsychological battery to assess cognitive function. Tests were overseen by a neuropsychologist and domain-specific T-scores were obtained by averaging within-domain demographically (age, sex, race/ethnicity, and education) corrected T-scores per administered tests.
Statistical analysis:
Descriptive statistics were performed as appropriate using Prism software (GraphPad; La Jolla, CA). Comparisons between the groups with undetectable and detectable Tat levels were made with two-way unpaired Student’s t-test for normally distributed continuous measurements and with Yates’ continuity corrected chi-square for binary variables.
RESULTS:
ART inhibits HIV replication in macrophages but does not diminish Tat protein expression.
Productive HIV infection in the brain is predominantly macrophage tropic [39]. Hence, we initially infected monocyte-derived macrophages (MDM) with HIVSF162 then treated with either a protease inhibitor, darunavir, or DMSO. Darunavir significantly reduced viral output compared to DMSO as measured by PERT assay (p = 0.0006) (Fig. 1a), but had no effect on levels of Tat protein as measured by ELISA (p = 0.758) (Fig. 1b). To determine whether ART has any effect on Tat levels in vivo, we collected CSF from five individuals prior to ART initiation, then again after two to five months of continuous treatment. Tat protein was elevated compared to pre-treatment levels in four patients at the follow-up appointment (Fig. 1c; UCSD cohort), which strongly suggests ART does not inhibit Tat expression and could potentially elicit an increase in Tat production or extend the half-life of Tat.
Figure 1.
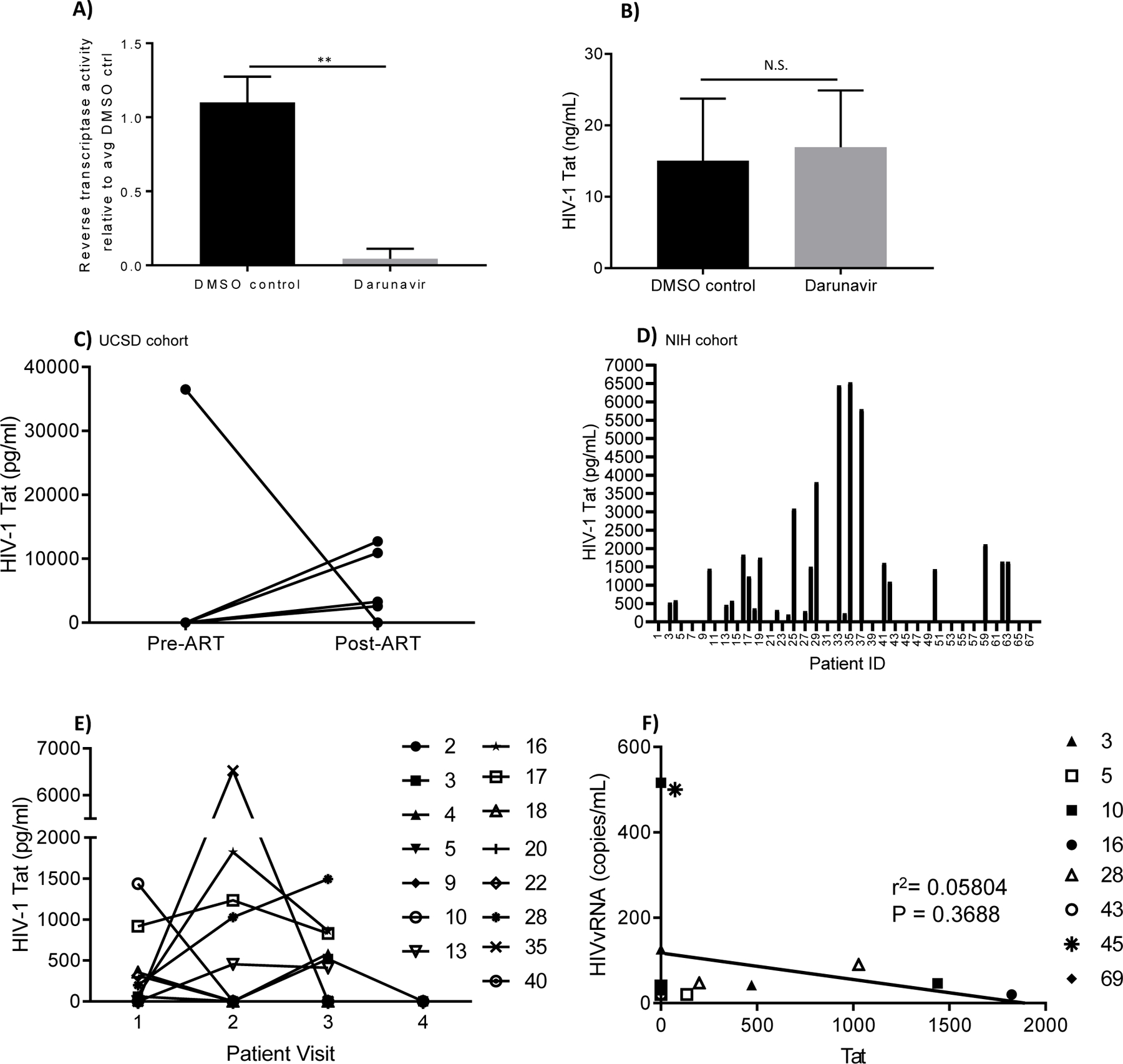
(A-B) Antiretroviral therapy (darunavir) inhibits viral release but does not diminish Tat protein expression in macrophages. Monocyte-derived macrophages (MDM) were infected with HIVSF162 followed by treatment with 1 uM darunavir or an equal volume of DMSO (vehicle). Medium was spiked with DMSO or darunavir every 48h hours. At 7 days post-infection supernatants were analyzed (A) for reverse transcriptase activity by a PERT assay and (B) cells were lysed for Tat by ELISA. Results indicate mean ± standard deviation from three independent experiments. **p<0.01 as determined by unpaired, two-tailed Student’s t-test; N.S. = not significant. (C-F) Presence of Tat protein in patients on antiretroviral therapy. Cerebrospinal fluid (C-F) was analyzed by ELISA for detection of Tat protein. Plots show (C) CSF Tat concentrations from five patients pre-ART initiation (Pre-ART) and two to five months after ART (Post-ART). (D) CSF Tat concentrations from 68 patients virologically well controlled on ART. (E) CSF Tat concentrations (pg/ mL) from 15 patients followed for at least three years. Samples were collected from patients at sequential yearly visits (Patient Visit). Data shown in (D) represent the highest CSF Tat level measured for that patient. For patients with longitudinal samples available, these data are repeated as a single time point in (E). (F) Correlation between Tat protein levels and HIV viral RNA (vRNA) in patients experiencing CNS viral escape without detectable virus in blood at the indicated time points. LOD = limit of detection for the ELISA (200 pg/ mL), as determined by background O.D. readings from 25 HIV-negative CSF samples + 2 standard deviations. All samples were run in triplicate with at least two negative controls per plate and quantitated relative to standard curve generated using recombinant Tat protein (rTat). Anti-Tat antibody specificity was validated by western blot using rTat and lysates from Tat-transfected or untransfected HEK293T/17.
Tat is detected in CSF despite long term ART.
Tat protein levels were measured by ELISA from CSF samples collected from 68 HIV positive patients virologically controlled with long-term ART. As shown in Fig. 1d, 36.8% (25/68) of patients had detectable Tat at levels ranging from 200 pg/ mL to 6.5 ng/ mL. Longitudinal CSF samples were available for 15 of the patients shown in fig. 1d, collected at 12- to 24-month intervals with at least three time points per patient. Ten of these patients (66.7%) had detectable Tat during at least one time point, with two patients (13.3%) positive for Tat at two consecutive time points and two patients (13.3%) positive at all three time points evaluated. Five patients (33.3%) were persistently Tat-negative at all three follow-up visits (Fig. 1e).
Tat levels are independent of CSF viral load.
Tat was measured from the CSF of eight patients who experienced transient CNS escape (CSF viral RNA ≥ 40 copies/mL but undetectable viral load in blood) during the course of the study. 3/8 (37.5%) patients were Tat-positive at the time of CNS viremia, but there was no correlation between CSF viral load and presence of Tat in spinal fluid (r2=0.058; p=0.37) (Fig. 1f and supplementary Table 1). Collectively, these results indicate that Tat is detectable in at least one-third of patients even in the absence of measurable HIV replication and that levels of secreted Tat fluctuate over time in the same individual.
Detection of Tat and TAR in CSF exosomes.
Several viral elements have been reported to be secreted in exosomes released from infected cells. These include TAR RNA [24], Nef [40], Gag [41] and Tat [27]. Exosomes containing biologically active, neurotoxic Tat were reported in Tat-expressing primary astrocytes and glioblastoma cells, and HIV-infected cell lines [27]. However, Tat has not been detected in exosomes from HIV-infected primary cells or isolated from bodily fluids [42]. To determine whether the Tat present in CSF is contained within exosomes, exosomes were isolated from CSF of 32 patients with HIV and two HIV-negative controls using Nanotrap particles, and western blot was performed to detect Tat protein. Exosomes from 11/32 patients (34.4%) contained significant amounts of Tat protein (Fig. 2a, c). Exosome lysates from an additional 13 patients (40.6%) were weakly positive for Tat (Fig. 2c, supplementary Fig. 2 and supplementary Table 2). Results from exosome capture correlated well with Tat ELISA in 20/23 (87%) of matched CSF samples, with an additional 2/23 (8.7%) positive on western blot but below the limit of detection by ELISA and only one sample (4.3%) weakly positive by ELISA only (supplementary Table 3). Interestingly, RNA extraction of exosomes showed that 10/32 (31%) patient-derived exosomes also contained trans-activation response element (TAR) RNA (Fig. 2b, c and supplementary Table 2), which indicates that HIV RNA is also packaged into CSF-circulating exosomes. The presence of TAR RNA was not closely correlated with Tat protein (Fig. 2c), suggesting that these viral elements may be packaged separately.
Figure 2. Presence of Tat protein and TAR RNA in exosomes isolated from CSF.
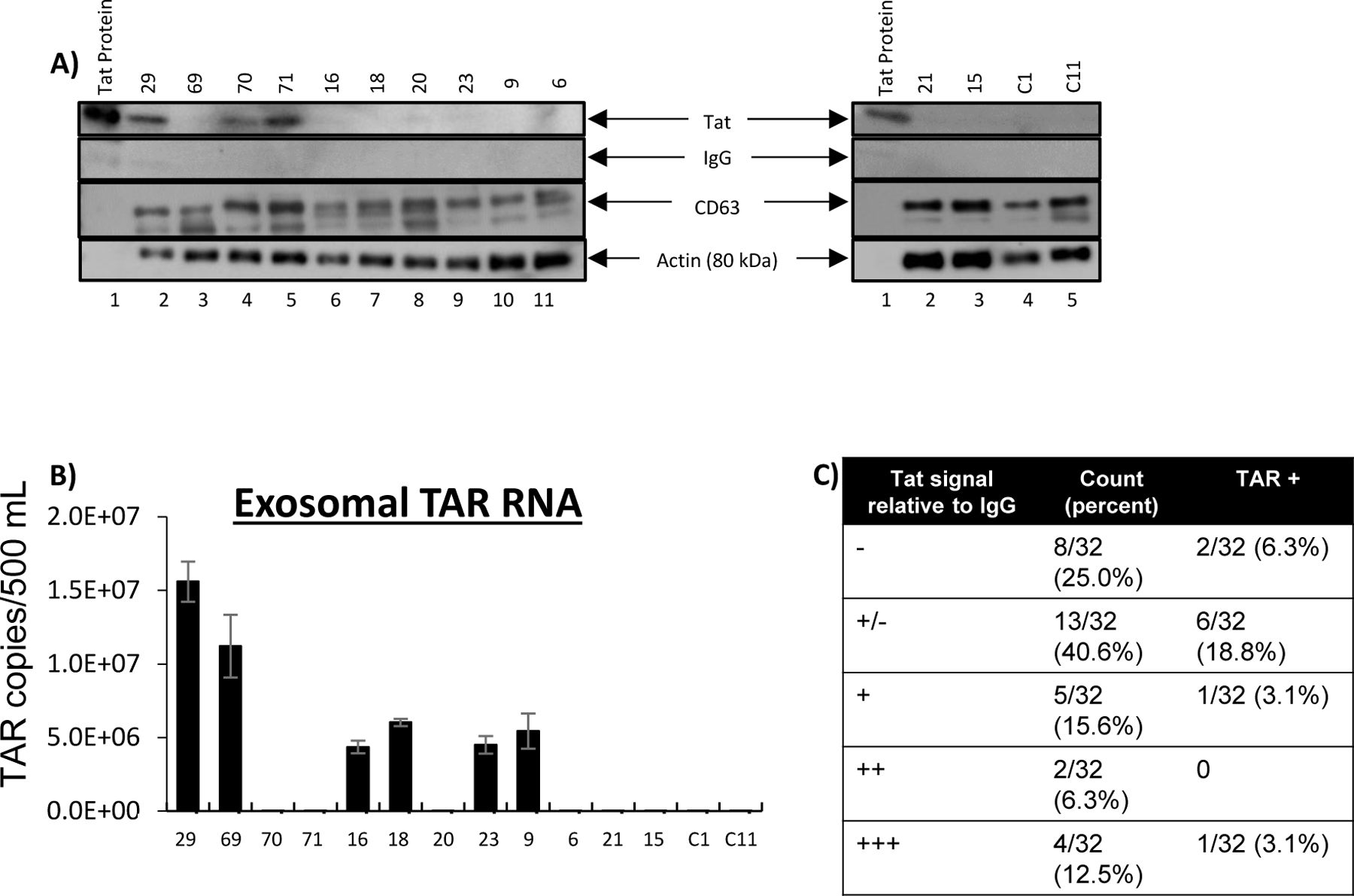
A) Immunoblot of isolated exosomes for the Tat protein, the exosome marker CD63, actin (loading control), and IgG (negative control) for background subtraction. The top of the image shows patient identification number and the bottom of the image indicates lane number.C1 and C11 are control samples from HIV seronegative patients. B) Levels of TAR RNA were measured in CSF exosomes by quantitative RT-PCR. Results indicate copies of TAR per 500 μl of CSF used for exosome isolation. Error bars represent ± S.D. of three technical replicates. C) Summary of exosome data showing number of patient samples positive for Tat protein and/or TAR RNA. Results shown in Tat column represent western blot densitometry relative to negative IgG control: - = <1%; +/− = 1%−14.99%; + = 15%−32.99%; ++ = 33%−65.99%; +++ = >66%. “TAR+” column indicates the number of patient samples positive for TAR RNA in each Tat category.
Tat in CSF is functionally active.
Tat is an intrinsically unfolded protein that is prone to aggregation and cleavage, with an intracellular half-life of approximately 2.6 hours [43, 44]. Since exosomes protect cargo against extracellular degradation, we evaluated whether exosome-derived Tat maintained transactivation activity. CEM-GFP cells, which contain an integrated HIV long-terminal repeat (LTR) linked to a GFP reporter, were exposed to CSF-derived exosomes from patients with detectable Tat levels or HIV-negative controls for 72 hours and GFP expression was evaluated by flow cytometry. As a positive control, CEM-GFP were infected with HIV (89.6; MOI:1.0) (Fig. 3). Exosomal Tat from 4/6 (66.7%) patients transactivated the LTR, at levels ranging from 28% to 68% compared to the level of induction by HIV infection (Fig. 3 and supplementary Table 4). Therefore, CSF Tat is functional in at least a subset of patients.
Figure 3. Tat protein isolated from CSF exosomes is capable of transactivating the HIV promoter.
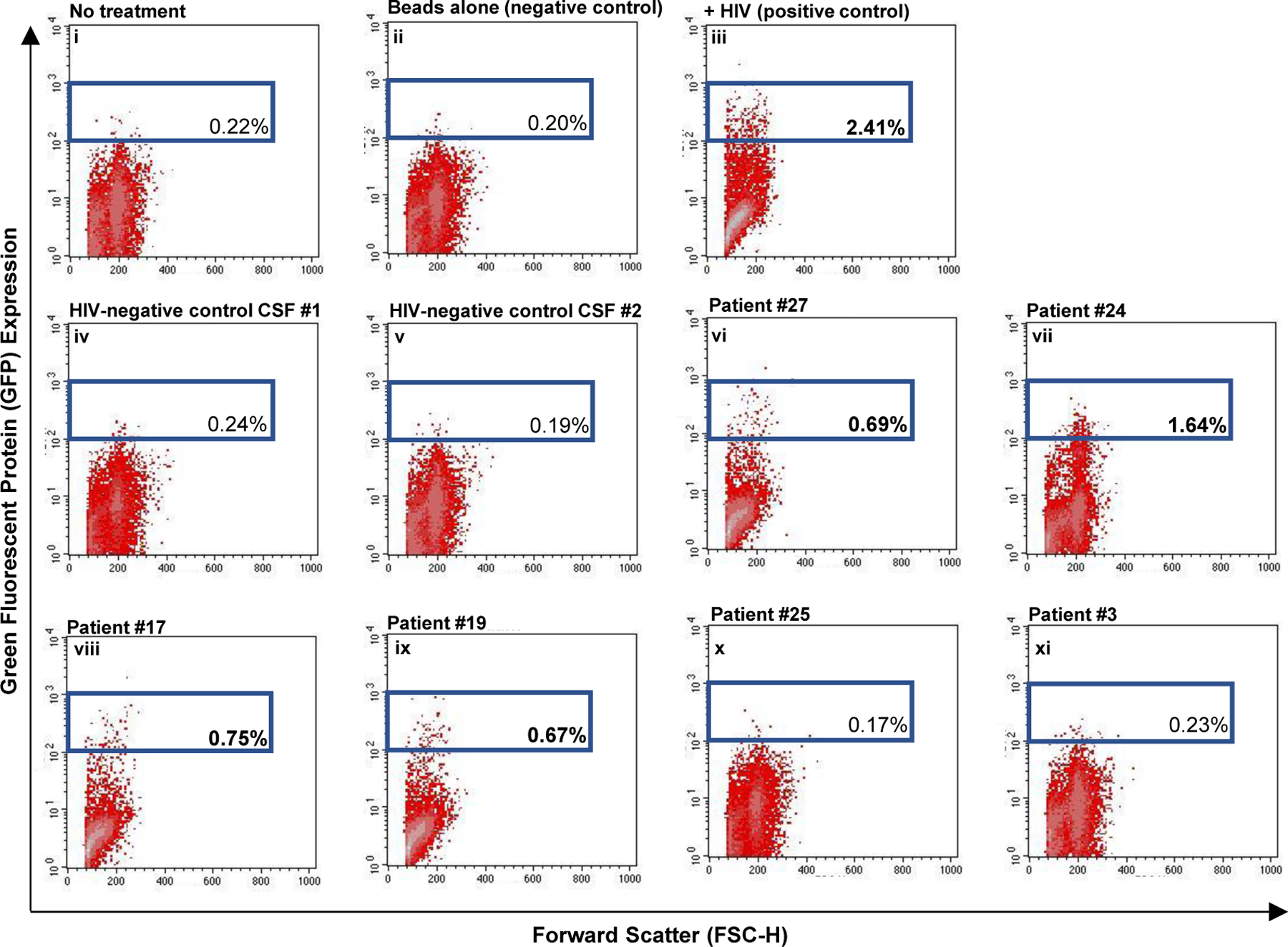
Scatter plots showing collected flow cytometry data. Side scatter is on the X-axis and GFP is on the y-axis. Exosomes from HIV-negative controls (control CSF #1, control CSF #2) or HIV-positive patients with detectable CSF Tat as measured by western blot (#27, #24, #17, #19, #25, #3) were directly added to CEM-GFP reporter cells, which contain a GFP gene under the control of an HIV promoter. 72 hours later, flow cytometry was used to detect GFP expression. As a positive control, cells were infected with HIV. Cells with no treatment or treatment with beads alone were used as negative controls. Sample ID is indicated in the top right corner and the gated number represents the percent of GFP-positive cells for a given sample. Bolded numbers indicated samples with gated events that exceed the negative control.
Presence of Tat in CSF correlates with history of drug abuse.
Substance abuse is a significant cofactor in HIV transmission and pathogenesis [45–48]. Therefore, we investigated the relationship between Tat levels and drug abuse. In our cohort, a history of drug abuse is common, with cocaine being the most commonly abused substance; 34.8% (23/66 on whom drug abuse history was available) met criteria for a history of cocaine abuse. Tat-positive patients were significantly more likely to have reported cocaine or stimulant (cocaine or amphetamine) abuse compared to those with undetectable Tat levels (p = 0.03, p = 0.01, respectively) (Fig. 4a, b). Participants who had ever used cocaine or stimulants, even without a history of abuse, were similarly more likely to have detectable CSF Tat (p = 0.02, p = 0.01, respectively. There were no similar associations with marijuana, tobacco or alcohol use or abuse and no associations with overall neurocognitive function; however, when measured by individual cognitive domain, psychomotor speed and information processing speed performance were lower in the group with detectable Tat (p = 0.04, p = 0.02, respectively). There were no differences in HIV-specific characteristics including time since diagnosis, time on antiretroviral therapy, antiretroviral regimen or time from diagnosis to therapy initiation (Fig. 4b).
DISCUSSION:
Collectively, our findings provide strong evidence that biologically active Tat and TAR RNA are present in the CSF of HIV patients on long-term ART. These viral products may provide a valuable measure of the CNS HIV reservoir. A proposed mechanism of the continued detection of Tat and TAR in the presence of antiretroviral therapy is included in Fig. 5. Briefly, in an HIV-infected cell that contains an actively transcribed provirus, protease inhibitors prevent cleavage of HIV polyproteins (Gag-Pol) to inhibit formation of new viral particles. However Tat, which does not require cleavage by the viral protease, may continue to be transcribed, translated and released from the cell as a secreted protein or within an exosome. TAR RNA, which is also transcribed under treated conditions, is also packaged into exosomes alone or in addition to Tat. Tat- and TAR-containing exosomes can then be endocytosed by other uninfected or HIV-infected cells to cause altered signaling. In HIV-infected cells that contain an inactive (latent) provirus, exogenous Tat may reactivate the latent provirus via induction of host signaling pathways such as NF-κB and transactivation of the HIV LTR.
Figure 5. Model depicting possible mechanisms of elevated Tat levels in the presence of antiretroviral therapy.
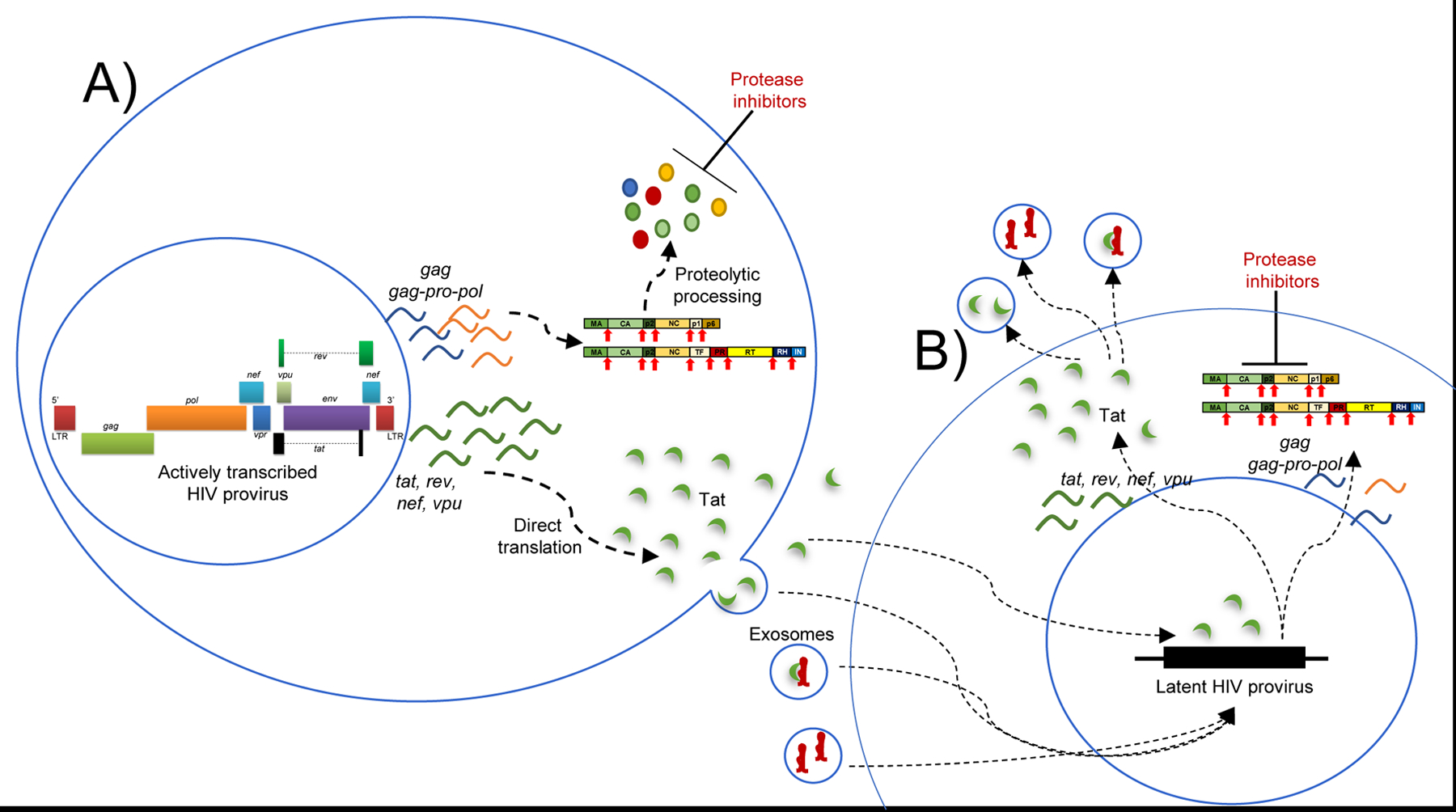
A) in a cell that contains an integrated, actively transcribed provirus, protease inhibitors prevent cleavage of HIV polyproteins (Gag-Pol), abrogating assembly and release of new virions. However Tat, which does not require cleavage by the viral protease, may continue to be transcribed, translated and released from the cell. Tat can be secreted directly into the extracellular space, or it may be packaged into exosomes. TAR RNA, which is also transcribed under treated conditions, is also packaged into exosomes alone or in addition to Tat. B) Tat- and TAR-containing exosomes can be endocytosed by other uninfected or HIV-infected cells to cause altered signaling. In HIV-infected cells which contain an inactive (latent) provirus, exogenous Tat can reactivate the latent provirus via induction of host signaling pathways such as NF-κB and transactivation of the HIV LTR. Productive replication is blocked in the newly transcribing cell due to the presence of protease inhibitors, but Tat protein and TAR RNA are produced and can be packaged into exosomes for delivery to other target cells.
Tat was detected in HIV-positive patients on prolonged ART as well as in a macrophage cell culture model of infection treated with an HIV protease inhibitor. As expected, we found that while darunavir inhibited HIV release, it had no effect on Tat production. We had previously shown that darunavir did not inhibit Tat production from HIV-infected lymphocytes in vitro, and that Tat could be detected in infiltrating leukocytes in the brain of an HIV patient on ART while immunostaining for p24 antigen was negative [15].
We found that at least 36.8% of patients had detectable Tat in the CSF by ELISA. Furthermore, enrichment of CSF exosomes using allowed for more sensitive detection of Tat from samples that were below the limit of detection by ELISA; up to 75% of patients had detectable Tat protein when samples were enriched for exosomes. Tat was present within the CSF despite prolonged viral suppression with ART (2–29 years), which suggests that Tat can be detected even in the absence of measurable replication and true latency of HIV is not achieved in the brain. CSF Tat levels did not correlate with viral load in patients with CNS escape. Additionally, in patients where Tat levels were measured before and after initiation of ART, no suppression of Tat was noted in most. It is worth noting that 2/5 ART-naïve patients were diagnosed with HAND at their pre-treatment visit (UCSD cohort; data not shown), including the single individual whose Tat level declined after treatment. Tat may therefore be a useful measure of the CNS HIV reservoir, although additional studies with larger sample sizes are needed to confirm these findings.
The fluctuation of Tat levels over time suggests that the CNS viral reservoir may be dynamic. Factors that influence viral activity in the CNS should be an area of active investigation. If Tat levels in CSF correlate with the active viral reservoir in the CNS, the effectiveness of cure strategies may be indirectly measured using Tat as a marker of actively transcribing proviruses. Furthermore, some of the Tat found in CSF is functional, as evidenced by its ability to transactivate the HIV LTR. This important finding suggests that anti-Tat therapeutics are needed to augment viral suppression in patients on ART.
Despite effective ART, many HIV-infected individuals have chronic, low-level inflammation and immune activation [49–51] that is strongly associated with increased morbidity and mortality [52]. Tat induces expression of pro-inflammatory cytokines and chemokines in vitro and in transgenic rodent models [10–14]. Indeed, Dickens and colleagues recently showed that “leaky” Tat expression in the tetracycline promoter-driven Tat transgenic (rtTA-Tat) mice was sufficient to cause neurodegeneration and neuroinflammation [53]. Another group showed that expression of Tat within the thymus in transgenic mice caused thymus atrophy, resulting in T-cell depletion and increased expression of NFκB-dependent cytokines and chemokines [54]. While a large body of experimental literature has revealed detrimental effects of Tat on brain and systemic organs, our ability to measure Tat in biological samples presents unique opportunities to determine the biological consequences of elevated Tat levels in HIV-positive patient populations.
We also determined that HIV TAR RNA could be detected in both Tat-positive and Tat-negative CSF exosomes. It is well known that the composition of exosome “cargo” is dependent on the cell type of origin, as well as other conditions such as cellular stress, viral infection and receptor-mediated signaling [55]. Exosomes have an important role in cell-to-cell signaling via delivery of transcription factors, mRNA, miRNA and other cellular constituents [55]. Exosomes are also important mediators of several viral infections, including HIV [56–62]; these viruses alter exosome contents to facilitate viral replication and transmission or promote tumorigenesis and disease pathogenesis. Pre-treatment of target cells with TAR-containing exosomes prior to HIV infection increased viral output by 1.5–4-fold, which strongly suggests that these particles can “prime” naïve cells for productive infection [24]. The detection of TAR from exosomes derived from the CNS of patients under ART suggests that TAR may also play a role in HIV-associated neuropathogenesis and may be a worthwhile target for adjunctive therapies.
Exosomes from some patients contained Tat protein alone, while others contained only TAR RNA or both Tat and TAR. Potentially, the Tat in exosomes may be modified (i.e. acetylated, methylated, ubiquitinated), which could allow Tat to have a longer half-life [63–65]. Alternatively, Tat may be found outside exosomes as multimers [66]. TAR-containing exosomes may be derived from various infected cell types. Exosomes from HIV-infected T-cells and myeloid cells have previously been shown to contain TAR [24, 25], but it is unknown whether exosomes from infected astrocytes and microglia also contain TAR. When both Tat and TAR are present within an exosome they may be bound together, potentially sequestering Tat from its intended target [67]. However, our current exosome capture method is incapable of differentiating between exosomes of different cell types, Tat multimers, and modified Tat proteins. It is also worth noting that the nanotrap particles used in this study, NT80 and NT82, are also capable of binding directly to Tat peptides even when they are not contained within an exosome [33]. Therefore, we cannot discount the possibility that unbound, free Tat is also detected by this method.
Finally, we found that HIV-positive individuals with a history of substance abuse, particularly the stimulants cocaine and amphetamine, were significantly more likely to have Tat-positive CSF. These findings are especially relevant because cocaine abuse is known to accelerate HIV disease progression [45, 68–77] and is an independent predictor of AIDS-related mortality [73]. Cocaine abuse contributes to oxidative stress and neuroinflammation in the brain [78–82]. Cocaine and other psychostimulants also compromise the integrity of the blood-brain barrier (BBB); in vitro studies indicate that cocaine disrupts brain microvascular endothelial cell (BMVEC) intercellular junctions and increases expression of endothelial adhesion molecules [79, 83–86], leading to enhanced transmigration of leukocytes across the BBB [81, 83, 86–89]. Cocaine also increases susceptibility of leukocytes [71, 90–92] and astrocytes [93] to HIV infection, impairs proliferation and effector function of immune cells [94–96] and enhances HIV replication in infected cells via activation of the NF-κB pathway and epigenetic remodeling of the HIV promoter [97–99].
It is worth noting that all patients were negative for cocaine use by urine screening at the beginning of this study. Several individuals (7/68) tested positive for cocaine at later time points, but there was no correlation between active drug use and current Tat levels in CSF (data not shown). Collectively, these findings suggest that use or abuse of psychostimulants at the time of HIV infection may enhance seeding of the CNS viral reservoir by multiple mechanisms including increased BBB permeability, enhanced susceptibility to infection and transmigration of leukocytes into the brain, reduced antiviral response via impaired effector function, and higher viral load during acute infection. Further research is required to better define the relationship between HIV infection, psychostimulant use and Tat expression, and between Tat levels and HIV burden in the brain.
We also observed a relationship between detection of Tat in CSF and worse performance in both psychomotor speed and information processing, two cognitive domains that rely primarily on subcortical integrity and those that are commonly associated with cognitive impairment attributable to HIV infection [100]. This is particularly important as a possible biomarker of cognitive impairment in HIV because it is virus-specific unlike other potential biomarkers including those measured in blood, CSF, or by MRI.
Our study has a few shortcomings. Our Tat assay may lack the sensitivity to measure the total amount of Tat in CSF, especially since Tat protein can polymerize, form immune complexes, and stick to collection and storage tubes. Our sample size was also not large enough to analyze the full biological effects of Tat on the patient population. Despite these shortcomings, this study provides evidence that ART is not sufficient to fully inhibit Tat expression from infected cells in the CNS, and some Tat may be sequestered in CSF exosomes and remains biologically active. Tat levels may reflect the size of the viral reservoir in the CNS and may be an important target for future drug development.
Supplementary Material
Supplementary Figure 1. Inter- and Intra-assay coefficients of variation (%CV) for the HIV-1 Tat ELISA. Tat ELISA was performed as described in the Materials and Methods. (A) The coefficient of variation (%CV) was determined individually for 20 CSF samples by calculating the mean and standard deviation (SD) for each sample run in duplicate, then dividing the SD of each sample by the duplicate mean and multiplying by 100. The inter-assay CV was then calculated by taking the average of the individual CVs (N=20). (B) The inter-assay %CV for the Tat ELISA from three independent runs performed in duplicate on different days. First, the plate means for the lowest and highest standards were calculated, then this value was averaged across runs (Mean of means). The standard deviation and %CV for each standard were calculated as described in (A), then the inter-assay %CV was determined by taking the average of the individual %CVs. (C) Calculated Tat protein values decrease uniformly with increasing dilution of an HIV-positive CSF sample. Tat ELISA was performed as described in the Materials and Methods using two-fold serial dilutions of a known Tat-positive CSF sample (#29) in blocking buffer. Results were calculated from the standard curve generated using recombinant Tat protein, without normalization by the dilution factor. Data represent results from a single ELISA plate run in triplicate.
Supplementary Figure 2. Representative Tat western blot performed on exosomes enriched from patient CSF. Exosomes were isolated and prepared for western blot as described in the Materials and Methods and were run in parallel with recombinant Tat protein (lane 1).
ACKNOWLEDGMENTS:
LJH and TPJ contributed to study design and interpretation of the data, performed experiments and statistical analysis and drafted the manuscript. FK and AN contributed to study design and interpretation of the data and edited the manuscript. RB and CD performed experiments, interpreted exosome data and performed statistical analysis. MB and JS generated recombinant Tat protein and assisted with interpretation of the data. SL, NS, and JM assisted with collection, storage and analysis of patient samples and data. UAS, BS and LBR assisted with collection and storage of patient samples and data, and assisted in interpretation of results. All authors read and approved the final manuscript.
Source of Funding:
This work was supported by the Intramural AIDS Research Fellowship, Office of Intramural Research, National Institutes of Health to LJH, AI078859, AI074410, AI127351-01, AI043894, and NS099029 to FK, 2P30MH075673-11A1 and 2P30AI094189-06 to JM, and intramural funds from the National Institute of Neurological Disorders and Stroke (NINDS) and OAR to AN.
Footnotes
Conflicts of Interest:
For the remaining authors none were declared.
Works Cited:
- 1.Deeks SG, Lewin SR, Havlir DV. The end of AIDS: HIV infection as a chronic disease. Lancet 2013; 382(9903):1525–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mothobi NZ, Brew BJ. Neurocognitive dysfunction in the highly active antiretroviral therapy era. Curr Opin Infect Dis 2012; 25(1):4–9. [DOI] [PubMed] [Google Scholar]
- 3.Heaton RK, Clifford DB, Franklin DR Jr., Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology 2010; 75(23):2087–2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berkhout B, Silverman RH, Jeang KT. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 1989; 59(2):273–282. [DOI] [PubMed] [Google Scholar]
- 5.Dingwall C, Ernberg I, Gait MJ, Green SM, Heaphy S, Karn J, et al. Human immunodeficiency virus 1 tat protein binds trans-activation-responsive region (TAR) RNA in vitro. Proc Natl Acad Sci U S A 1989; 86(18):6925–6929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones KA. HIV trans-activation and transcription control mechanisms. New Biol 1989; 1(2):127–135. [PubMed] [Google Scholar]
- 7.Robinson R A simple feedback resistor switch keeps latent HIV from awakening. PLoS Biol 2007; 5(1):e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rayne F, Debaisieux S, Yezid H, Lin YL, Mettling C, Konate K, et al. Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J 2010; 29(8):1348–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Couret J, Chang TL. Reactive Oxygen Species in HIV Infection. EC Microbiol 2016; 3(6):597–604. [PMC free article] [PubMed] [Google Scholar]
- 10.Ambrosino C, Ruocco MR, Chen X, Mallardo M, Baudi F, Trematerra S, et al. HIV-1 Tat induces the expression of the interleukin-6 (IL6) gene by binding to the IL6 leader RNA and by interacting with CAAT enhancer-binding protein beta (NF-IL6) transcription factors. J Biol Chem 1997; 272(23):14883–14892. [DOI] [PubMed] [Google Scholar]
- 11.Buscemi L, Ramonet D, Geiger JD. Human immunodeficiency virus type-1 protein Tat induces tumor necrosis factor-alpha-mediated neurotoxicity. Neurobiol Dis 2007; 26(3):661–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen P, Mayne M, Power C, Nath A. The Tat protein of HIV-1 induces tumor necrosis factor-alpha production. Implications for HIV-1-associated neurological diseases. J Biol Chem 1997; 272(36):22385–22388. [DOI] [PubMed] [Google Scholar]
- 13.Nath A, Conant K, Chen P, Scott C, Major EO. Transient exposure to HIV-1 Tat protein results in cytokine production in macrophages and astrocytes. A hit and run phenomenon. J Biol Chem 1999; 274(24):17098–17102. [DOI] [PubMed] [Google Scholar]
- 14.Zidovetzki R, Wang JL, Chen P, Jeyaseelan R, Hofman F. Human immunodeficiency virus Tat protein induces interleukin 6 mRNA expression in human brain endothelial cells via protein kinase C- and cAMP-dependent protein kinase pathways. AIDS Res Hum Retroviruses 1998; 14(10):825–833. [DOI] [PubMed] [Google Scholar]
- 15.Johnson TP, Patel K, Johnson KR, Maric D, Calabresi PA, Hasbun R, et al. Induction of IL-17 and nonclassical T-cell activation by HIV-Tat protein. Proc Natl Acad Sci U S A 2013; 110(33):13588–13593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eugenin EA, King JE, Nath A, Calderon TM, Zukin RS, Bennett MV, et al. HIV-tat induces formation of an LRP-PSD-95- NMDAR-nNOS complex that promotes apoptosis in neurons and astrocytes. Proc Natl Acad Sci U S A 2007; 104(9):3438–3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li W, Li G, Steiner J, Nath A. Role of Tat protein in HIV neuropathogenesis. Neurotox Res 2009; 16(3):205–220. [DOI] [PubMed] [Google Scholar]
- 18.Magnuson DS, Knudsen BE, Geiger JD, Brownstone RM, Nath A. Human immunodeficiency virus type 1 tat activates non-N-methyl-D-aspartate excitatory amino acid receptors and causes neurotoxicity. Ann Neurol 1995; 37(3):373–380. [DOI] [PubMed] [Google Scholar]
- 19.Zhou BY, Liu Y, Kim B, Xiao Y, He JJ. Astrocyte activation and dysfunction and neuron death by HIV-1 Tat expression in astrocytes. Mol Cell Neurosci 2004; 27(3):296–305. [DOI] [PubMed] [Google Scholar]
- 20.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 2013; 113(1):1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwab A, Meyering SS, Lepene B, Iordanskiy S, van Hoek ML, Hakami RM, et al. Extracellular vesicles from infected cells: potential for direct pathogenesis. Front Microbiol 2015; 6:1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta 2012; 1820(7):940–948. [DOI] [PubMed] [Google Scholar]
- 23.Fleming A, Sampey G, Chung MC, Bailey C, van Hoek ML, Kashanchi F, et al. The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog Dis 2014; 71(2):109–120. [DOI] [PubMed] [Google Scholar]
- 24.Narayanan A, Iordanskiy S, Das R, Van Duyne R, Santos S, Jaworski E, et al. Exosomes derived from HIV-1-infected cells contain trans-activation response element RNA. J Biol Chem 2013; 288(27):20014–20033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sampey GC, Saifuddin M, Schwab A, Barclay R, Punya S, Chung MC, et al. Exosomes from HIV-1-infected Cells Stimulate Production of Pro-inflammatory Cytokines through Trans-activating Response (TAR) RNA. J Biol Chem 2016; 291(3):1251–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Demarino C, Pleet M, Cowen M, Barclay R, Akpamagbo Y, Erickson J, et al. Antiretroviral Drugs Alter the Content of Extracellular Vesicles from HIV-1 Infected Cells. Sci Rep 2018; Accepted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rahimian P, He JJ. Exosome-associated release, uptake, and neurotoxicity of HIV-1 Tat protein. J Neurovirol 2016; 22(6):774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturdevant CB, Joseph SB, Schnell G, Price RW, Swanstrom R, Spudich S. Compartmentalized replication of R5 T cell-tropic HIV-1 in the central nervous system early in the course of infection. PLoS Pathog 2015; 11(3):e1004720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuda K, Brown CR, Foley B, Goeken R, Whitted S, Dang Q, et al. Laser capture microdissection assessment of virus compartmentalization in the central nervous systems of macaques infected with neurovirulent simian immunodeficiency virus. J Virol 2013; 87(16):8896–8908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valcour V, Chalermchai T, Sailasuta N, Marovich M, Lerdlum S, Suttichom D, et al. Central nervous system viral invasion and inflammation during acute HIV infection. J Infect Dis 2012; 206(2):275–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vermeire J, Naessens E, Vanderstraeten H, Landi A, Iannucci V, Van Nuffel A, et al. Quantification of reverse transcriptase activity by real-time PCR as a fast and accurate method for titration of HIV, lenti- and retroviral vectors. PLoS One 2012; 7(12):e50859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson TP, Nath A. Protocol for Detection of HIV-Tat Protein in Cerebrospinal Fluid by a Sandwich Enzyme-Linked Immunosorbent Assay. Methods Mol Biol 2016; 1354:343–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jaworski E, Saifuddin M, Sampey G, Shafagati N, Van Duyne R, Iordanskiy S, et al. The use of Nanotrap particles technology in capturing HIV-1 virions and viral proteins from infected cells. PLoS One 2014; 9(5):e96778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Busby E, Whale AS, Ferns RB, Grant PR, Morley G, Campbell J, et al. Instability of 8E5 calibration standard revealed by digital PCR risks inaccurate quantification of HIV DNA in clinical samples by qPCR. Sci Rep 2017; 7(1):1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Folks TM, Powell D, Lightfoote M, Koenig S, Fauci AS, Benn S, et al. Biological and biochemical characterization of a cloned Leu-3- cell surviving infection with the acquired immune deficiency syndrome retrovirus. J Exp Med 1986; 164(1):280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilburn KM, Mwandumba HC, Jambo KC, Boliar S, Solouki S, Russell DG, et al. Heterogeneous loss of HIV transcription and proviral DNA from 8E5/LAV lymphoblastic leukemia cells revealed by RNA FISH:FLOW analyses. Retrovirology 2016; 13(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hauber J, Perkins A, Heimer EP, Cullen BR. Trans-activation of human immunodeficiency virus gene expression is mediated by nuclear events. Proc Natl Acad Sci U S A 1987; 84(18):6364–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gervaix A, West D, Leoni LM, Richman DD, Wong-Staal F, Corbeil J. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc Natl Acad Sci U S A 1997; 94(9):4653–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Budka H Neuropathology of human immunodeficiency virus infection. Brain Pathol 1991; 1(3):163–175. [DOI] [PubMed] [Google Scholar]
- 40.Campbell TD, Khan M, Huang MB, Bond VC, Powell MD. HIV-1 Nef protein is secreted into vesicles that can fuse with target cells and virions. Ethn Dis 2008; 18(2 Suppl 2):S2–14–19. [PMC free article] [PubMed] [Google Scholar]
- 41.Booth AM, Fang Y, Fallon JK, Yang JM, Hildreth JE, Gould SJ. Exosomes and HIV Gag bud from endosome-like domains of the T cell plasma membrane. J Cell Biol 2006; 172(6):923–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li M, Aliotta JM, Asara JM, Tucker L, Quesenberry P, Lally M, et al. Quantitative proteomic analysis of exosomes from HIV-1-infected lymphocytic cells. Proteomics 2012; 12(13):2203–2211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gargano B, Fiorillo M, Amente S, Majello B, Lania L. p14ARF is capable of promoting HIV-1 tat degradation. Cell Cycle 2008; 7(10):1433–1439. [DOI] [PubMed] [Google Scholar]
- 44.Sivakumaran H, van der Horst A, Fulcher AJ, Apolloni A, Lin MH, Jans DA, et al. Arginine methylation increases the stability of human immunodeficiency virus type 1 Tat. J Virol 2009; 83(22):11694–11703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cofrancesco J Jr., Scherzer R, Tien PC, Gibert CL, Southwell H, Sidney S, et al. Illicit drug use and HIV treatment outcomes in a US cohort. AIDS 2008; 22(3):357–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Friedman H, Pross S, Klein TW. Addictive drugs and their relationship with infectious diseases. FEMS Immunol Med Microbiol 2006; 47(3):330–342. [DOI] [PubMed] [Google Scholar]
- 47.Kipp AM, Desruisseau AJ, Qian HZ. Non-injection drug use and HIV disease progression in the era of combination antiretroviral therapy. J Subst Abuse Treat 2011; 40(4):386–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sohler NL, Wong MD, Cunningham WE, Cabral H, Drainoni ML, Cunningham CO. Type and pattern of illicit drug use and access to health care services for HIV-infected people. AIDS Patient Care STDS 2007; 21 Suppl 1:S68–76. [DOI] [PubMed] [Google Scholar]
- 49.Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, et al. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis 2011; 204(8):1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Pablo-Bernal RS, Ruiz-Mateos E, Rosado I, Dominguez-Molina B, Alvarez-Rios AI, Carrillo-Vico A, et al. TNF-alpha levels in HIV-infected patients after long-term suppressive cART persist as high as in elderly, HIV-uninfected subjects. J Antimicrob Chemother 2014; 69(11):3041–3046. [DOI] [PubMed] [Google Scholar]
- 51.Erlandson KM, Allshouse AA, Jankowski CM, Lee EJ, Rufner KM, Palmer BE, et al. Association of functional impairment with inflammation and immune activation in HIV type 1-infected adults receiving effective antiretroviral therapy. J Infect Dis 2013; 208(2):249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Deeks SG, Tracy R, Douek DC. Systemic effects of inflammation on health during chronic HIV infection. Immunity 2013; 39(4):633–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dickens AM, Yoo SW, Chin AC, Xu J, Johnson TP, Trout AL, et al. Chronic low-level expression of HIV-1 Tat promotes a neurodegenerative phenotype with aging. Sci Rep 2017; 7(1):7748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Fiume G, Scialdone A, Albano F, Rossi A, Tuccillo FM, Rea D, et al. Impairment of T cell development and acute inflammatory response in HIV-1 Tat transgenic mice. Sci Rep 2015; 5:13864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Samanta S, Rajasingh S, Drosos N, Zhou Z, Dawn B, Rajasingh J. Exosomes: new molecular targets of diseases. Acta Pharmacol Sin 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ahsan NA, Sampey GC, Lepene B, Akpamagbo Y, Barclay RA, Iordanskiy S, et al. Presence of Viral RNA and Proteins in Exosomes from Cellular Clones Resistant to Rift Valley Fever Virus Infection. Front Microbiol 2016; 7:139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Guenat D, Hermetet F, Pretet JL, Mougin C. Exosomes and Other Extracellular Vesicles in HPV Transmission and Carcinogenesis. Viruses 2017; 9(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jaworski E, Narayanan A, Van Duyne R, Shabbeer-Meyering S, Iordanskiy S, Saifuddin M, et al. Human T-lymphotropic virus type 1-infected cells secrete exosomes that contain Tax protein. J Biol Chem 2014; 289(32):22284–22305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kulkarni R, Prasad A. Exosomes Derived from HIV-1 Infected DCs Mediate Viral trans-Infection via Fibronectin and Galectin-3. Sci Rep 2017; 7(1):14787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Meckes DG Jr., Gunawardena HP, Dekroon RM, Heaton PR, Edwards RH, Ozgur S, et al. Modulation of B-cell exosome proteins by gamma herpesvirus infection. Proc Natl Acad Sci U S A 2013; 110(31):E2925–2933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Pleet ML, Mathiesen A, DeMarino C, Akpamagbo YA, Barclay RA, Schwab A, et al. Ebola VP40 in Exosomes Can Cause Immune Cell Dysfunction. Front Microbiol 2016; 7:1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ramakrishnaiah V, Thumann C, Fofana I, Habersetzer F, Pan Q, de Ruiter PE, et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma Huh7.5 cells. Proc Natl Acad Sci U S A 2013; 110(32):13109–13113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Deng L, de la Fuente C, Fu P, Wang L, Donnelly R, Wade JD, et al. Acetylation of HIV-1 Tat by CBP/P300 increases transcription of integrated HIV-1 genome and enhances binding to core histones. Virology 2000; 277(2):278–295. [DOI] [PubMed] [Google Scholar]
- 64.Kumar S, Maiti S. The effect of N-acetylation and N-methylation of lysine residue of Tat peptide on its interaction with HIV-1 TAR RNA. PLoS One 2013; 8(10):e77595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang L, Qin J, Li Y, Wang J, He Q, Zhou J, et al. Modulation of the stability and activities of HIV-1 Tat by its ubiquitination and carboxyl-terminal region. Cell Biosci 2014; 4(1):61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hategan A, Bianchet MA, Steiner J, Karnaukhova E, Masliah E, Fields A, et al. HIV Tat protein and amyloid-beta peptide form multifibrillar structures that cause neurotoxicity. Nat Struct Mol Biol 2017; 24(4):379–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Richter S, Cao H, Rana TM. Specific HIV-1 TAR RNA loop sequence and functional groups are required for human cyclin T1-Tat-TAR ternary complex formation. Biochemistry 2002; 41(20):6391–6397. [DOI] [PubMed] [Google Scholar]
- 68.Anthony JC, Vlahov D, Nelson KE, Cohn S, Astemborski J, Solomon L. New evidence on intravenous cocaine use and the risk of infection with human immunodeficiency virus type 1. Am J Epidemiol 1991; 134(10):1175–1189. [DOI] [PubMed] [Google Scholar]
- 69.Baum MK, Rafie C, Lai S, Sales S, Page B, Campa A. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr 2009; 50(1):93–99. [DOI] [PubMed] [Google Scholar]
- 70.Buch S, Yao H, Guo M, Mori T, Mathias-Costa B, Singh V, et al. Cocaine and HIV-1 interplay in CNS: cellular and molecular mechanisms. Curr HIV Res 2012; 10(5):425–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chaisson RE, Bacchetti P, Osmond D, Brodie B, Sande MA, Moss AR. Cocaine use and HIV infection in intravenous drug users in San Francisco. JAMA 1989; 261(4):561–565. [PubMed] [Google Scholar]
- 72.Cook JA. Associations between use of crack cocaine and HIV-1 disease progression: research findings and implications for mother-to-infant transmission. Life Sci 2011; 88(21–22):931–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, et al. Crack cocaine, disease progression, and mortality in a multicenter cohort of HIV-1 positive women. AIDS 2008; 22(11):1355–1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Larrat EP, Zierler S, Mayer K. Cocaine use and HIV disease progression among heterosexuals. Pharmacoepidemiol Drug Saf 1996; 5(4):229–236. [DOI] [PubMed] [Google Scholar]
- 75.Nath A, Hauser KF, Wojna V, Booze RM, Maragos W, Prendergast M, et al. Molecular basis for interactions of HIV and drugs of abuse. J Acquir Immune Defic Syndr 2002; 31 Suppl 2:S62–69. [DOI] [PubMed] [Google Scholar]
- 76.Siddiqui NS, Brown LS Jr., Makuch RW. Short-term declines in CD4 levels associated with cocaine use in HIV-1 seropositive, minority injecting drug users. J Natl Med Assoc 1993; 85(4):293–296. [PMC free article] [PubMed] [Google Scholar]
- 77.Nath A, Maragos WF, Avison MJ, Schmitt FA, Berger JR. Acceleration of HIV dementia with methamphetamine and cocaine. J Neurovirol 2001; 7(1):66–71. [DOI] [PubMed] [Google Scholar]
- 78.Clark KH, Wiley CA, Bradberry CW. Psychostimulant abuse and neuroinflammation: emerging evidence of their interconnection. Neurotox Res 2013; 23(2):174–188. [DOI] [PubMed] [Google Scholar]
- 79.Fiala M, Eshleman AJ, Cashman J, Lin J, Lossinsky AS, Suarez V, et al. Cocaine increases human immunodeficiency virus type 1 neuroinvasion through remodeling brain microvascular endothelial cells. J Neurovirol 2005; 11(3):281–291. [DOI] [PubMed] [Google Scholar]
- 80.Fox HC, D’Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, et al. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol 2012; 27(2):156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gan X, Zhang L, Berger O, Stins MF, Way D, Taub DD, et al. Cocaine enhances brain endothelial adhesion molecules and leukocyte migration. Clin Immunol 1999; 91(1):68–76. [DOI] [PubMed] [Google Scholar]
- 82.Walker J, Winhusen T, Storkson JM, Lewis D, Pariza MW, Somoza E, et al. Total antioxidant capacity is significantly lower in cocaine-dependent and methamphetamine-dependent patients relative to normal controls: results from a preliminary study. Hum Psychopharmacol 2014; 29(6):537–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Dhillon NK, Peng F, Bokhari S, Callen S, Shin SH, Zhu X, et al. Cocaine-mediated alteration in tight junction protein expression and modulation of CCL2/CCR2 axis across the blood-brain barrier: implications for HIV-dementia. J Neuroimmune Pharmacol 2008; 3(1):52–56. [DOI] [PubMed] [Google Scholar]
- 84.Kousik SM, Napier TC, Carvey PM. The effects of psychostimulant drugs on blood brain barrier function and neuroinflammation. Front Pharmacol 2012; 3:121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sharma HS, Muresanu D, Sharma A, Patnaik R. Cocaine-induced breakdown of the blood-brain barrier and neurotoxicity. Int Rev Neurobiol 2009; 88:297–334. [DOI] [PubMed] [Google Scholar]
- 86.Yao H, Duan M, Buch S. Cocaine-mediated induction of platelet-derived growth factor: implication for increased vascular permeability. Blood 2011; 117(8):2538–2547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fiala M, Gan XH, Zhang L, House SD, Newton T, Graves MC, et al. Cocaine enhances monocyte migration across the blood-brain barrier. Cocaine’s connection to AIDS dementia and vasculitis? Adv Exp Med Biol 1998; 437:199–205. [DOI] [PubMed] [Google Scholar]
- 88.Gandhi N, Saiyed ZM, Napuri J, Samikkannu T, Reddy PV, Agudelo M, et al. Interactive role of human immunodeficiency virus type 1 (HIV-1) clade-specific Tat protein and cocaine in blood-brain barrier dysfunction: implications for HIV-1-associated neurocognitive disorder. J Neurovirol 2010; 16(4):294–305. [DOI] [PubMed] [Google Scholar]
- 89.Yao H, Kim K, Duan M, Hayashi T, Guo M, Morgello S, et al. Cocaine hijacks sigma1 receptor to initiate induction of activated leukocyte cell adhesion molecule: implication for increased monocyte adhesion and migration in the CNS. J Neurosci 2011; 31(16):5942–5955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roth MD, Tashkin DP, Choi R, Jamieson BD, Zack JA, Baldwin GC. Cocaine enhances human immunodeficiency virus replication in a model of severe combined immunodeficient mice implanted with human peripheral blood leukocytes. J Infect Dis 2002; 185(5):701–705. [DOI] [PubMed] [Google Scholar]
- 91.Roth MD, Whittaker KM, Choi R, Tashkin DP, Baldwin GC. Cocaine and sigma-1 receptors modulate HIV infection, chemokine receptors, and the HPA axis in the huPBL-SCID model. J Leukoc Biol 2005; 78(6):1198–1203. [DOI] [PubMed] [Google Scholar]
- 92.Napuri J, Pilakka-Kanthikeel S, Raymond A, Agudelo M, Yndart-Arias A, Saxena SK, et al. Cocaine enhances HIV-1 infectivity in monocyte derived dendritic cells by suppressing microRNA-155. PLoS One 2013; 8(12):e83682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Reynolds JL, Mahajan SD, Bindukumar B, Sykes D, Schwartz SA, Nair MP. Proteomic analysis of the effects of cocaine on the enhancement of HIV-1 replication in normal human astrocytes (NHA). Brain Res 2006; 1123(1):226–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baldwin GC, Tashkin DP, Buckley DM, Park AN, Dubinett SM, Roth MD. Marijuana and cocaine impair alveolar macrophage function and cytokine production. Am J Respir Crit Care Med 1997; 156(5):1606–1613. [DOI] [PubMed] [Google Scholar]
- 95.Klein TW, Matsui K, Newton CA, Young J, Widen RE, Friedman H. Cocaine suppresses proliferation of phytohemagglutinin-activated human peripheral blood T-cells. Int J Immunopharmacol 1993; 15(1):77–86. [DOI] [PubMed] [Google Scholar]
- 96.Mao JT, Huang M, Wang J, Sharma S, Tashkin DP, Dubinett SM. Cocaine down-regulates IL-2-induced peripheral blood lymphocyte IL-8 and IFN-gamma production. Cell Immunol 1996; 172(2):217–223. [DOI] [PubMed] [Google Scholar]
- 97.Tyagi M, Bukrinsky M, Simon GL. Mechanisms of HIV Transcriptional Regulation by Drugs of Abuse. Curr HIV Res 2016; 14(5):442–454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Tyagi M, Weber J, Bukrinsky M, Simon GL. The effects of cocaine on HIV transcription. J Neurovirol 2016; 22(3):261–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sahu G, Farley K, El-Hage N, Aiamkitsumrit B, Fassnacht R, Kashanchi F, et al. Cocaine promotes both initiation and elongation phase of HIV-1 transcription by activating NF-kappaB and MSK1 and inducing selective epigenetic modifications at HIV-1 LTR. Virology 2015; 483:185–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychol Rev 2009; 19(2):152–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Inter- and Intra-assay coefficients of variation (%CV) for the HIV-1 Tat ELISA. Tat ELISA was performed as described in the Materials and Methods. (A) The coefficient of variation (%CV) was determined individually for 20 CSF samples by calculating the mean and standard deviation (SD) for each sample run in duplicate, then dividing the SD of each sample by the duplicate mean and multiplying by 100. The inter-assay CV was then calculated by taking the average of the individual CVs (N=20). (B) The inter-assay %CV for the Tat ELISA from three independent runs performed in duplicate on different days. First, the plate means for the lowest and highest standards were calculated, then this value was averaged across runs (Mean of means). The standard deviation and %CV for each standard were calculated as described in (A), then the inter-assay %CV was determined by taking the average of the individual %CVs. (C) Calculated Tat protein values decrease uniformly with increasing dilution of an HIV-positive CSF sample. Tat ELISA was performed as described in the Materials and Methods using two-fold serial dilutions of a known Tat-positive CSF sample (#29) in blocking buffer. Results were calculated from the standard curve generated using recombinant Tat protein, without normalization by the dilution factor. Data represent results from a single ELISA plate run in triplicate.
Supplementary Figure 2. Representative Tat western blot performed on exosomes enriched from patient CSF. Exosomes were isolated and prepared for western blot as described in the Materials and Methods and were run in parallel with recombinant Tat protein (lane 1).


