Abstract
Heparan sulphate (HS) represents a heterogeneous class of molecules on cell membranes and extracellular matrices. These molecules are involved in a variety of biological processes, including immune responses, through their binding and functional modulation of proteins. Recently a panel of HS-epitope–specific, human single chain antibodies have been generated by phage display, facilitating analysis of the structural heterogeneity of HS in relation to pathological conditions. In a pilot study a heterogeneous staining pattern in melanoma metastases was observed with one of the clones (EW4G1). Using a double-staining technique, the expression of this epitope was studied in 12 metastatic melanoma lesions in relation to the presence of a CD3+ cell infiltrate. Different staining patterns with EW4G1 were observed in the different lesions. The different staining patterns were associated with the presence and pattern of inflammation with CD3+ cells. A pronounced staining pattern of blood vessels with EW4G1 was associated with a more or less brisk presence of CD3+ cells, while a pronounced staining of tumour cells or tumour cell matrix or absence of staining with EW4G1 was associated with absence of CD3+ cells. These results suggest a dualistic role for HS in the recruitment and intratumoural migration of CD3+ cells, depending on the location of expression of its epitope recognized by EW4G1. Further characterization of the structural diversity of HS and its function in T-cell recruitment and migration is therefore warranted, since detailed understanding of this relation may provide new targets for therapeutic intervention, such that better homing and migration of T cells (in)to tumours might be achieved in immunologically based treatment strategies.
Keywords: Heparan sulphate, T-cell recruitment, Inflammation, Melanoma
Introduction
Heparan sulphate (HS) represents a heterogeneous class of molecules within the group of glycosaminoglycans and is associated with cell membranes and extracellular matrices. HS binds and modulates a variety of biological molecules such as extracellular matrix proteins, cytokines, adhesion molecules, degradative enzymes and protease inhibitors [5]. HS has also been shown to be involved in leukocyte adhesion and function and has been implicated in tumour cell invasion [14]. The different functional features of HS seem to be linked to the amount and location of its sulphate groups [11, 4, 9]. Until recently it was difficult to study in detail this structural and functional diversity of HS in relation to pathological conditions. Due to the general lack of antigenicity of HS molecules, epitope-specific antibodies have been extremely limited. Recently a panel of human single chain antibodies directed against different epitopes of HS has been generated [13, 12]. In a pilot study, tissue sections from human melanoma metastases were stained with these antibodies. With one of the single chain antibody clones (EW4G1) variable staining patterns in different metastatic lesions were observed. Given the significant role of HS in immune responses [10] and the implicated role of HS in melanoma formation [1, 7], we studied the expression of this HS epitope in different metastatic melanoma lesions in relation to the inflammatory response.
Material and methods
Histological material
Surgical biopsy material from 12 patients with metastatic melanoma was used (University Hospital, Linköping, Sweden). Immediately after resection, biopsy samples were snap-frozen and stored at −70°C until further processing. Frozen, 6–7-μm-thick tissue sections were used for immunohistochemical analysis.
Human single chain antibodies
The anti-HS single chain clone EW4G1 and the irrelevant non-HS-binding single chain clone TSC01 were produced as described previously [12](also, Smetsers et al., in preparation), and affinity-purified using protein-A agarose columns as described by Ey et al. [2]. These single chain antibodies were characterized previously [12]. Some characteristics are summarized in Table 1.
Table 1.
Summary of characteristics of the single chain antibodies used in this study. Indicated are the antibody code, amino acid sequence of the VH complementary-determining region 3 (CDR3), VH family and germ line segment (DP numbering). Hep heparin, HS heparin sulphate
| Antibody code | CDR3 | VH | DP | Reactivity In ELISA |
|---|---|---|---|---|
| EW4G1 | GARLKR | 3 | 42 | Hep, HSa |
| TSCO1 | LGFHS | 3 | 40 | -b |
aEW4G1 does not react with chondroitin sulfate and does show a moderate anticoagulant effect of heparin
bTSCO1 does not show any reactivity with glycosaminoglycans
Immunohistochemistry
The staining procedure consisted of 5 min fixation of cryosections in 4% paraformaldehyde, followed subsequently by 15 min blocking with 10% human AB serum, incubation with anti-CD3 (Dako, Stockholm, Sweden) for 30 min, washing with BSS, incubation with Envision mouse HRP (Dako) for 30 min, washing in Hank's balanced salt solution (BSS, Gibco, Paisley, United Kingdom) and in Tris-HCL (pH=8.2), substrate reaction with DAB, washing in Tris-HCL and in BSS, blocking with 1% BSA in BSS for 15 min, incubation with EW4G1 for 40 min, washing in BSS, incubation with the mouse anti-VSV antibody (P5D4, Sigma,) for 30 min, washing in BSS, incubation with Envision (Dako), washing in BSS and Tris-HCl, substrate reaction with Fast red (Sigma), washing in Tris-HCl, incubation in Mayer's haematoxylin, and washing in Tris-HCl. All incubations were performed at room temperature. Controls for specific binding of EW4G1 and anti-CD3 consisted of a nonrelevant non-HS-binding single chain antibody TSC01 and IgG1 control respectively.
Results
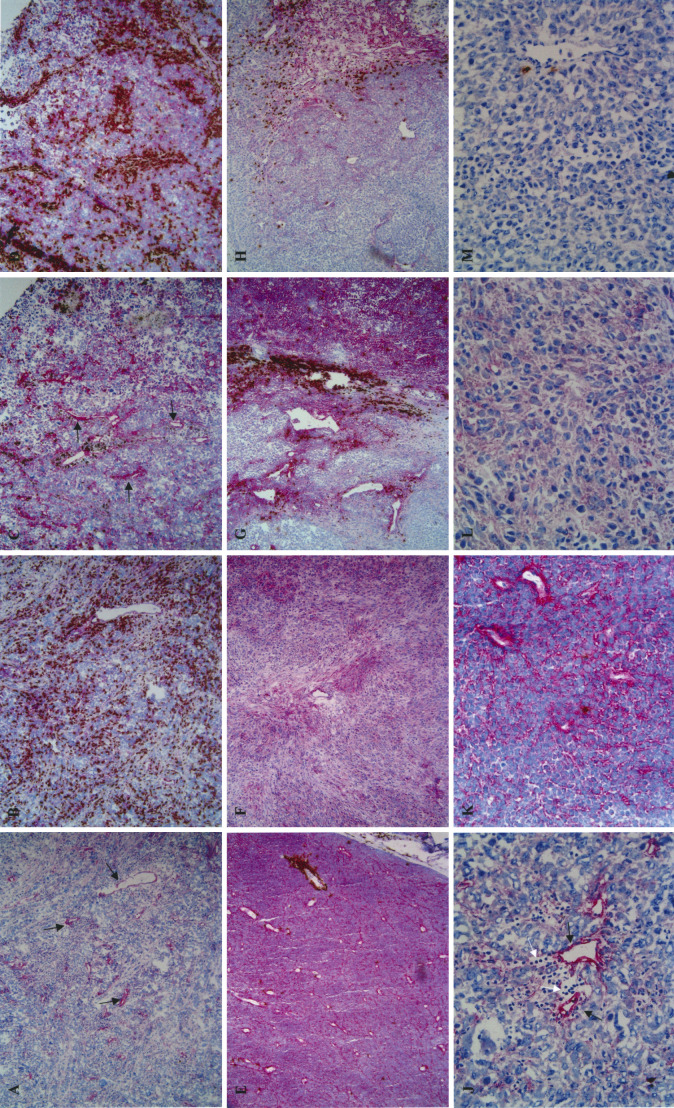
Immunohistochemical staining with EW4G1 resulted in three basic staining patterns, i.e. a pronounced staining of blood vessels, a pronounced staining of tumour cells and/or tumour cell matrix or no staining (Fig. 1). The different staining patterns were associated with the presence and pattern of inflammation with CD3+ cells. A pronounced staining pattern of blood vessels with EW4G1 was associated with a more or less abundant, brisk CD3+ cell infiltrate (Fig. 1A–D, J). In contrast, a pronounced staining of tumour cells or tumour cell matrix (overall staining pattern) or absence of staining with EW4G1 was associated with absence of CD3+ cells (Fig. 1E–F, K–M). These patterns were found in varying degrees in the various lesions. A predominant presence of the vascular staining pattern in association with a brisk infiltrate was found in 2 out of the 12 lesions. Three out of the 12 lesions showed a predominant presence of an overall staining pattern associated with an absence of a CD3+ cell infiltrate. One lesion was almost completely negative for the EW4G1 epitope and was without the presence of a CD3+ cell infiltrate except for an occasional CD3+ cell. The remaining six lesions showed two or all three staining patterns as exemplified in Fig. 1G, H. In Fig. 1G a transitional area is shown containing all three staining patterns. On the right side of the picture is an area with an overall staining pattern with EW4G1containing no or hardly any CD3+ cells. On the left side of the picture is an area negative for EW4G1 also containing no or hardly any CD3+ cells. Between these two areas, an area with pronounced vascular staining is seen that does contain CD3+ cells.
Fig. 1A–M.
Staining patterns of frozen tissue sections from malignant melanoma lesions with the anti-HS human single chain antibody clone EW4G1 and anti-CD3. A Overview (×20 objective) of a pronounced vascular staining (red; as indicated by arrows) with EW4G1. B Overview (×20 objective) of a double staining with EW4G1 (red) and anti-CD3 (brown) showing the association between a vascular staining with EW4G1 and a brisk presence of CD3+ cells in the same area of the same lesion as shown in A. In the double staining, the staining with EW4G1 becomes less pronounced. C Similar to A, from another lesion from another patient. D Similar to B, but for the lesion shown in C. E Overview (×20 objective) of a double staining with EW4G1 (red) and anti-CD3 (brown) showing an overall-staining pattern with EW4G1 without the presence of CD3+ cells (except for one small group surrounding one vessel). F Similar to E, from another lesion from another patient without the presence of CD3+ cells. G Overview (×20 objective) of a double staining with EW4G1 (red) and anti-CD3 (brown) showing a heterogeneous staining pattern within one lesion. H Similar to G, from another lesion from another patient. J Detailed view (×40 objective) of a pronounced vascular staining (red, indicated by black arrows) with EW4G1. Note the associated presence of (unstained) lymphocytes indicated by white arrows. K Detailed view (×40 objective) of an overall-staining pattern (red) with EW4G1. Note the absence of associated lymphocytes. L Similar to K, from another lesion from another patient. M Detailed view (×40 objective) of a negative staining pattern with EW4G1. Note the absence of associated lymphocytes
Discussion
Heparan sulphate chains have been shown to be involved in a number of biological processes through the binding of a variety of biologic effector molecules. Different biological functions of HS appear to be associated with different positions of the sulphate groups, which act as specific binding sites for proteins [6, 11]. In this report, an association of an HS epitope recognized by the human single chain antibody clone EW4G1 and the presence of a CD3+ infiltrate in malignant melanoma is demonstrated for the first time. A pronounced expression of this epitope on blood vessels was associated with the presence of a brisk infiltrate, while a pronounced expression of this epitope on tumour cells or the tumour cell matrix, or absence of expression, was associated with an absence of a brisk infiltrate. These results suggest a dualistic role for HS in the recruitment and intratumoural migration of CD3+ cells, depending on the location of expression of the epitope recognized by EW4G1. In vitro studies have shown that HS on endothelial cells bind and modulate the function of a variety of chemokines thus affecting the recruitment of cells to sites of inflammation [8, 9, 10]. It is therefore conceivable that expression of the epitope recognized by EW4G1 on endothelial cells is a prerequisite for recruitment of CD3+ cells as observed in this study. However, abundant expression of this epitope on tumour cells or the tumour cell matrix seems to abrogate this effect and seems to hamper migration of CD3+ cells. This may be due to competitive binding of chemokines, or different function modulation of chemokines by HS expressed on tumour cells. Herschkovic et al. [3] have shown that disaccharides enzymatically generated from HS could abrogate T-cell adhesion and chemotactic migration. We think that further characterization of the structural diversity of HS and its function in T-cell recruitment and migration is warranted. Detailed understanding of this relation may provide new targets for therapeutic intervention, such that better homing and migration of T cells (in)to tumours might be achieved in immunologically based treatment strategies.
Acknowledgements
The authors wish to thank Marie Jadner, Karin Hellander and Catharina Tranaeus Röckert for excellent technical help in performing the immunocyto- and immunohistochemistry stainings. This research was supported by grants from the Dutch Cancer Society (grant numbers 96-1366 and 98-1801), the County Council of Östergötland and the Health Research Council in south-east Sweden.
References
- 1.Caux Biochem Soc Trans. 1990;18:293. doi: 10.1042/bst0180293. [DOI] [PubMed] [Google Scholar]
- 2.Ey Immunochemistry. 1978;15:429. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 3.Hershkoviz Immunology. 2000;99:87. doi: 10.1046/j.1365-2567.2000.00931.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kato J Biol Chem. 1994;269:18881. [PubMed] [Google Scholar]
- 5.Lindahl J Biol Chem. 1998;273:24979. doi: 10.1074/jbc.273.39.24979. [DOI] [PubMed] [Google Scholar]
- 6.Lortat-Jacob Cell Mol Biol. 1991;37:253. [PubMed] [Google Scholar]
- 7.Moczar Clin Exp Metastasis. 1993;11:462. doi: 10.1007/BF00054937. [DOI] [PubMed] [Google Scholar]
- 8.Nathan J Cell Biol. 1991;113:981. doi: 10.1083/jcb.113.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Salmivirta FASEB J. 1996;10:1270. doi: 10.1096/fasebj.10.11.8836040. [DOI] [PubMed] [Google Scholar]
- 10.Selvan Ann N Y Acad Sci. 1996;797:127. doi: 10.1111/j.1749-6632.1996.tb52955.x. [DOI] [PubMed] [Google Scholar]
- 11.Turnbull J Biol Chem. 1992;267:10337. [PubMed] [Google Scholar]
- 12.van Blood. 2002;99:2427. doi: 10.1182/blood.V99.7.2427. [DOI] [PubMed] [Google Scholar]
- 13.van J Biol Chem. 1998;273:12960. doi: 10.1074/jbc.273.21.12960. [DOI] [PubMed] [Google Scholar]
- 14.Vlodavsky Cancer Metastasis Rev. 1990;9:203. doi: 10.1007/BF00046361. [DOI] [PubMed] [Google Scholar]