Abstract
Dendritic cells (DCs) are one of the most potent antigen-presenting cells (APCs) capable of activating immune responses. Different forms of tumor antigens have been used to load DCs to initiate tumor-specific immune responses. Heat shock proteins (HSPs) are considered natural adjuvants which have the ability to chaperone peptides associated with them presented efficiently by interaction with professional APCs through specific receptors. In the present study, we used HSP, gp96-peptide complexes, derived from human hepatocellular carcinoma (HCC) cells as antigens for pulsing DCs. We found that gp96-peptide complexes derived from HCC cells induced the maturation of DCs by enhancing expression of human leukocyte antigen class II, CD80, CD86, CD40, and CD83. The matured DCs stimulated a high level of autologous T cell proliferation and induced HCC specific cytotoxic T lymphocytes, which specifically killed HCC cells by a major histocompatability complex (MHC) class I restricted mechanism. These findings demonstrate that DCs pulsed with gp96-peptide complexes derived from HCC cells are effective in activating specific T cell responses against HCC cells.
Keywords: Cytotoxic T lymphocytes, Dendritic cells, Gp96-peptide complexes, Heat shock protein, Hepatocelluar carcinoma
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant diseases in China, and it rarely responds to conventional treatment such as surgery, radiation or chemotherapy [23, 35]. Although encouraging advances have been made in the past few decades, there are still many difficulties in treating patients with advanced stage HCC and in preventing recurrence and metastasis [23]. The ability of tumors to escape from immune surveillance contributes to their uncontrolled growth, recurrence and metastasis [25]. Immunotherapy, which tends to stimulate tumor-specific immune responses, is an active, positive and promising antitumor therapeutic method.
Heat shock proteins (HSPs) are important chaperones which include many members and are ubiquitously expressed at a basal level but are specifically induced in response to various stress conditions such as heat, anoxia and metabolic stress. HSPs mediate a range of essential housekeeping and cytoprotective functions [27, 28]. In recent years, studies have demonstrated that HSP-peptide complexes extracted from tumor cells can elicit specific antitumor immune responses against the tumor from which they are derived. The observed immunogenicity of HSP preparations is derived from the antigenic peptides chaperoned by HSPs [9, 20, 21, 33, 38]. Studies concerning the mechanism of the immunogenicity of these HSP-peptide complexes have shown that HSPs transfer antigenic peptides to professional antigen-presenting cells (APCs), such as dendritic cells (DCs), through receptors and channel them into the MHC class I antigen-presenting pathway, to activate antigen-specific T cells [2, 7, 10, 30, 31, 36]. HSPs can also elicit cytokine production by, and adhesion molecule expression of, a range of cell types, and they can deliver maturation signals to APCs through receptor-mediated interactions [2, 14, 28]. These functions suggest that HSPs could be immunoregulatory agents with potent and widely applicable therapeutic uses. HSP-peptide complexes from tumors are potent sources of tumor antigens, including both identified and unidentified tumor-associated antigens, which are able to be presented by professional APCs with high efficiency through the interaction of HSPs and their receptors.
Dendritic cells are extremely efficient APCs, capable of inducing both primary and secondary immune responses. DCs express MHC class I, class II, costimulatory and adhesion molecules which are necessary for stimulation of naïve T cell populations. These cells reside in an immature state in nonlymphoid tissues, where they efficiently capture and process antigens. After antigen uptake, they migrate to the secondary lymphoid tissues where they mature and stimulate T cells with decreased antigen-processing ability and enhanced expression of MHC and costimulatory molecules [4, 6, 19]. Studies have shown that DCs-based vaccines can induce potent antitumor immunity [15]. Various strategies have been developed to introduce tumor-specific antigens into DCs and thereby to generate cytotoxic T lymphocyte (CTL) responses against malignant cells. DCs pulsed with tumor-associated antigens or transduced with tumor-specific genes have led to the induction of antitumor immunity, but such strategies are restricted to identified tumor-associated antigens in the context of the particular human leukocyte antigen (HLA) subclass and the necessary costimulatory molecules [11]. Approaches designed to circumvent such restrictions include loading of DCs with tumor-cell lysates, peptides eluted from tumor-cell membranes, or tumor-cell RNA, as well as fusion of DCs with tumor cells [3, 18, 44]. Such strategies can induce antitumor immunity against multiple tumor antigens, including both identified and unidentified antigens.
In the present study, we used DCs pulsed with human HSP, gp96-peptide complexes, derived from human HCC cell line SMMC-7721 to activate autologous T cells. The results showed that DCs pulsed with the gp96-peptide complexes were functionally active in stimulating autologous T cell proliferation and inducing SMMC-7721 specific CTLs. The CTLs were directed against tumor-specific antigens and exhibited MHC class I restricted cytotoxicity. The gp96-peptide complexes also induced the maturation of DCs by enhancing expression of HLA class II, CD80, CD86, CD83 and CD40. Our results indicate that the specific immunogenic peptides, complexed by human HSP, gp96, were presented to T cells effectively by DCs. Our current research studied a potentially efficient immunotherapeutic approach which used DCs pulsed with human HSP, gp96-peptide complexes. We found the complexes were able to induce multiple antitumor immune responses without the need for prior identification of tumor-associated antigens. These findings could provide a rationale for HSP-based vaccination against human HCC.
Materials and methods
Culture of cell lines
Human HCC SMMC-7721 [16] and BEL-7402 [43] cell lines, and NK cell sensitive cell line K562 were cultured in RPMI 1640 medium(Gibco-BRL, CA, USA) supplemented with 10% heat-inactivated fetal calf serum (FCS) (HyClone, UT, USA), 2 mM L-glutamine (Gibco-BRL), 100 units/ml penicillin G, 100 μg/ml streptomycin and 250 ng/ml amphotericin B (Gibco-BRL) and incubated in 5% CO2 at 37°C.
Preparation of DCs and T cells
The DCs were generated as described with some modifications [18, 39]. Briefly, peripheral blood mononuclear cells (PBMCs) were isolated from healthy donors (Beijing Red Cross Blood Center, China) by Ficoll-Hypaque (1.077 g, Shang Hai Heng Xin Co., China) density gradient centrifugation and cultured in RPMI 1640 medium containing 10% FCS for 2 h. The nonadherent cells were removed for isolation of T cells and the adherent cells were cultured for 6 days in RPMI 1640 medium containing 10% FCS, 1,000 units/ml hGM-CSF (Biosea Biotechnology Co., China) and 500 units/ml hIL-4 (Peprotech Inc., NJ, USA). Culture medium and cytokines were refreshed every other day. DCs were harvested from the nonadherent and loosely adherent cells.
T cells were purified by nylon wool column (Polysciences Inc., Warrington, USA) from nonadherent cells according to manufacturer’s instructions. Briefly, nonadherent cells were resuspended in RPMI 1640 medium containing 10% FCS and added to the prepared nylon wool column. After incubating for 1 h at 37°C, the nonadherent cells, which contained mainly T cells, were collected by washing with RPMI 1640 medium containing 10% FCS.
Purification of gp96-peptide complexes from Human HCC SMMC-7721
The method for purification of human gp96 was as described with some modifications [32, 34]. In brief, SMMC-7721 tumor cells were harvested by culturing tumor cells in roller bottles and then 10 g tumor cells were suspended in 40 ml (four volumes) of 30 mM sodium bicarbonate, pH 7.0, and lysed by Dounce homogenization. The homogenate was centrifuged at 100,000 g for 90 min at 4°C and the supernatant obtained. After two steps of ammonium sulfate precipitation, 50% saturation followed by 80% saturation, the precipitate was solubilized and subjected to column chromatography using affinity chromatography Con A Sepharose, Sephadex G25 for buffer exchange, and ion-exchange chromatography with DEAE-Sepharose (all from Amersham Pharmacia Biotech, NJ, USA). To further purify the gp96-peptide complexes, the eluted proteins were subjected to AKTAFPLC system (Amersham Pharmacia Biotech, NJ, USA) using ion-exchange chromatography HiTrap Q followed by gel filtration chromatography Superdex 75. The obtained proteins were then identified by 12% reducing SDS-PAGE followed by silver staining and immunoblotting with anti-human gp96 antibody (Medical& Biological Laboratories, Japan). Fractions containing gp96 as the major protein were stored at −20°C and used for additional experiments. Protein concentration was determined by Bio-Rad protein assay (Bio-Rad Laboratories, Inc., CA, USA). Endotoxin level in the preparations was determined by limulus amebocyte lysate (LAL) assay (Ocean Biologicals Co., China). The contamination level of Con A in the fractions was tested by immunoblotting with anti-Con A antibody (Vector Laboratories, Inc., Burlingame, USA) and Con A protein was used as a positive control.
Expression and purification of human gp96 from E.coli
Human gp96 expression vector pET30a-gp96 (kindly provided by Professor Tian Bo) was constructed by inserting the complete coding sequence of human gp96 into prokaryotic expression vector pET30a. The recombinant human gp96 was expressed in E. coli as His-tagged protein and purified with affinity chromatography Ni-NTA agarose (Novagen, Scotland, UK) followed by ion-exchange chromatography DEAE-Sepharose. The eluted proteins were further purified with AKTAFPLC system using ion-exchange chromatography HiTrap Q followed by gel filtration chromatography Superdex 75. The purified proteins were identified by reducing 12% SDS-PAGE followed by silver staining. Endotoxin level in the preparations was determined by LAL assay.
T cell proliferation assay
The DCs were pulsed with the gp96-peptide complexes purified from SMMC-7721 cells at 37°C for 4 h and then harvested followed by washing with PBS. Autologous T cells isolated by nylon wool column were then cocultured with DCs in 96-well flat bottom culture plate (NUNC, Roskilde, Denmark). Proliferation was determined after 5 days by uptake of tritiated thymidine (Amersham Pharmacia Biotech, Buckinghamshire, England) measured at 18 h after a pulse of 1 μCi per well.
In vitro cytotoxicity test
The DCs were pulsed with the gp96-peptide complexes purified from SMMC-7721 cells or recombinant human gp96 isolated from E. coli at 37°C for 4 h. After washing with PBS, DCs were then cocultured with autologous T cells isolated by nylon wool column for 7–10 days in the presence of 20 units/ml human IL-2 (Kexing Co., China) in 24-well culture plate (NUNC, Roskilde, Denmark). The stimulated T cells were harvested with Ficoll-Hypaque density gradient centrifugation to remove the dead cell debris and used as effector cells in the CTL assay using LDH cytotoxicity detection kit (Roche, Indianapolis, USA). SMMC-7721, BEL-7402, K562 and autologous DCs pulsed with or without human gp96-peptide complexes were used as target cells in the assay. Briefly, target cells and effector cells were resuspended in assay medium (RPMI 1640 with 1%BSA), and then target cells (104 cells per well) were cocultured with effector cells at different ratios in 96-well round bottom culture plate (NUNC, Roskilde, Denmark) at 37°C. In the indicated experiment, target cells were preincubated with anti-MHC class I antibody (W6/32, PharMingen, San Diego, USA) for 30 min at 37°C before being cocultured with effector cells to test if the cytotoxicity was MHC class I restricted. After 5 h of incubation, the culture plates were centrifuged and the supernantant (100 μl per well) was transferred to another ELISA plate (NUNC, Roskilde, Denmark). Hundred microliter per well LDH detection mixture was then added and incubated in the dark for 30 min at room temperature. After adding 50 μl stop solution per well, the absorbance of the samples was measured by ELISA reader (Bio-Rad Laboratories, Inc. CA, USA) at 490 nm with 630 nm as reference wavelength. The spontaneous release of LDH by target cells or effector cells was assessed by incubation of target cells in the absence of effector cells and vice versa. The maximum release of LDH was determined by incubation of target cells in 1% Triton X-100 in assay medium. The percentage of specific cell mediated cytotoxicity was determined by the following equation: specific cytotoxicity (%) = [(effector&target mixture-effector spontaneous-target spontaneous)/(maximum-target spontaneous)]x100.
Flow cytometry
The DCs pulsed with human gp96-peptide complexes or unpulsed DCs were washed with cold PBS and incubated with murine antibodies directed against HLA–DR, CD80, CD86, CD83, CD40 (all from PharMingen), for 1 h on ice. After washing with cold PBS, the cells were incubated with FITC conjugated with goat anti-mouse IgG (Zymed Laboratories, Inc., South San Francisco, USA) for 30 min on ice. The cells were then washed with cold PBS and fixed with 2% paraformaldehyde. The fluorescence intensity was analyzed by FACS Calibur and the CellQuest software (Becton Dickinson, NJ, USA).
Results
Purification of gp96-peptide complexes from Human HCC SMMC-7721 and expression of recombinant human gp96 from E. coli
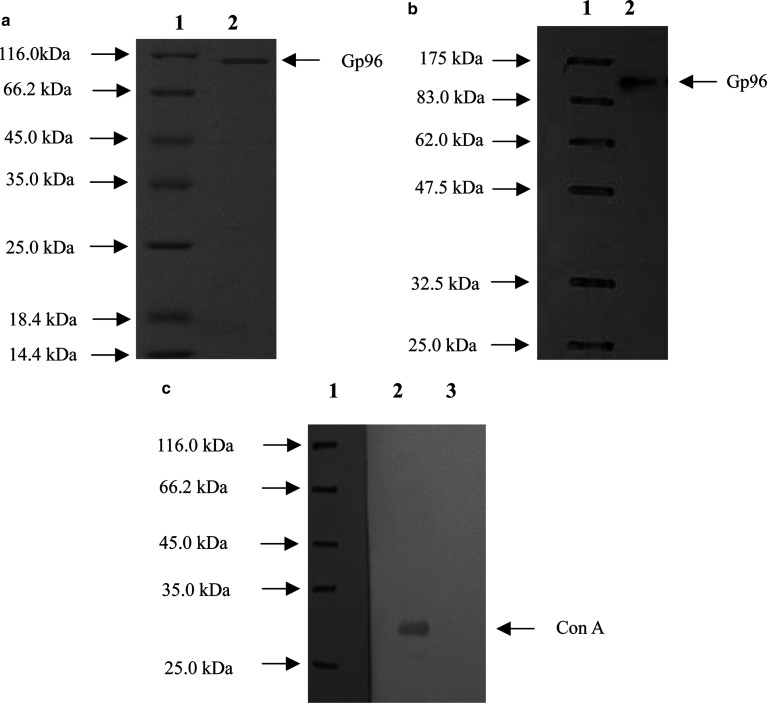
Gp96-peptide complexes were purified from cultured HCC cell line SMMC-7721 cells as described in “Materials and methods”. The purified protein was identified by reducing SDS-PAGE followed by silver staining and immunoblotting with human gp96 specific antibody. The result indicated that the protein obtained after purification was approximately 96 kDa (Fig. 1a) and was recognized by anti-human gp96 antibody (Fig. 1b), demonstrating that the eluted protein was human gp96. The purity of the gp96-peptide complex preparation was >99% as estimated by silver stained SDS-PAGE; no other stained bands could be observed on the gel. The endotoxin level in the preparations was lower than 0.03 Eu/μg of the gp96-peptide complexes as determined by LAL assay. To test if there was contaminating Con A in the preparations, the obtained proteins were immunoblotted with a Con A-specific antibody (Vector Laboratories). As shown in Fig. 1c, in contrast to the positive control of Con A protein, the gp96-peptide complexes were not recognized by the anti-Con A antibody, indicating that the contamination level of Con A was below the testing limit of immunoblotting.
Fig. 1.
Purification of gp96-peptide complexes from human HCC cell line SMMC-7721. a 12% reducing SDS-PAGE analysis of purified gp96-peptide complexes with silver staining. Gp96-peptide complexes were isolated from SMMC-7721 cells as described in Materials and methods; b immunoblotting analysis of purified gp96-peptide complexes. Gp96-peptide complexes were identified by anti- human gp96 antibody. Lane 1 represents the protein molecular marker and lane 2 represents human gp96-peptide complexes; c analysis of Con A contamination level in human gp96-peptide complexes by immunoblotting. Lane 1 represents the protein molecular marker, lane 2 represents positive control of Con A protein (10 ng) and lane 3 represents human gp96-peptide complexes (2 μg)
The recombinant human gp96 was expressed in the form of soluble protein and purified from E. coli. The purity of recombinant gp96 preparation was >99% as estimated by silver stained SDS-PAGE (data not shown) and the endotoxin level was lower than 0.03 Eu/μg of recombinant gp96 as determined by LAL assay.
DCs pulsed with gp96-peptide complexes derived from SMMC-7721 cells stimulate autologous T cell proliferation
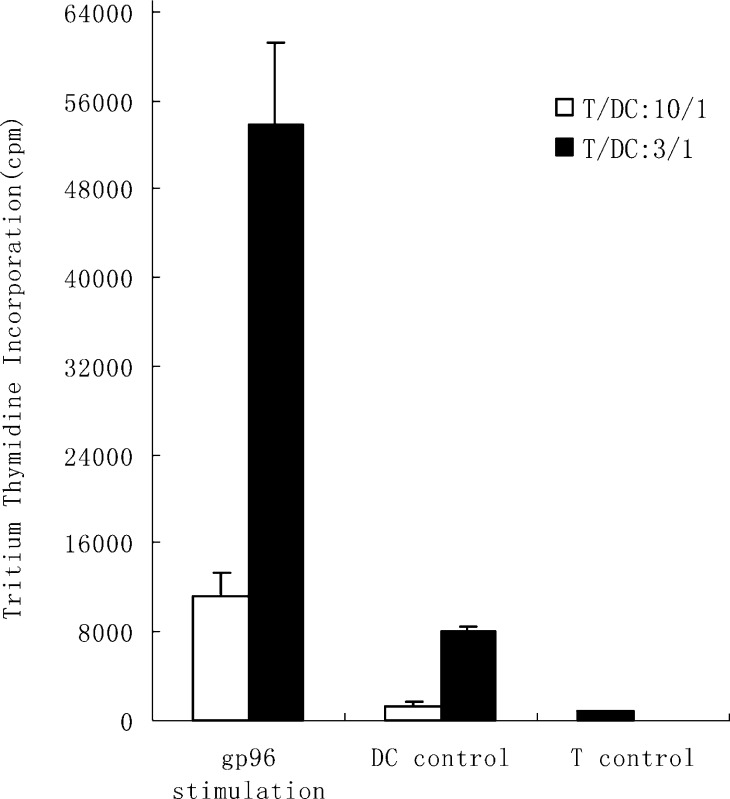
To determine if DCs pulsed with gp96-peptide complexes derived from SMMC-7721 cells were effective in stimulating autologous T cell proliferation, the autologous T cells, isolated by nylon wool column from nonadherent PBMCs of healthy donors, were cocultured with DCs, which had been incubated with human gp96-peptide complexes for 4 h at 37°C. After a 5-day coculture, the T cell proliferation was tested by the uptake of tritiated thymidine. As a control, the T cells were used alone or were cocultured with unpulsed DCs. The results showed that DCs pulsed with the gp96-peptide complexes had huge T cell simulation activity and by contrast, unpulsed DCs had very little stimulatory effect on autologous T cells. As shown in Fig. 2, T cells cocultured with autologous DCs pulsed with the gp96-peptide complexes at the ratio of T/DC: 10/1 proliferated tenfold compared with those cultured with unpulsed DCs and up to 14 times more than those cultured alone. Increasing the ratio of DCs:T cells in coculture rapidly enhanced the T cell proliferation. When the ratio of DCs:T cells was 1/3, T cells cocultured with unpulsed DCs proliferated to some extent, although at much lower rate than DCs pulsed with gp96-peptide complexes. This proliferation may have resulted from the immunogenicity of FCS, as we used FCS instead of human serum to culture DCs. But T cells cocultured with unpulsed DCs could not kill SMMC-7721 cells, whereas those cultured with the DCs pulsed with human gp96-peptide complexes could (as shown below). These findings indicate that DCs pulsed with human gp96-peptide complexes are capable of efficiently stimulating autologous T cell proliferation.
Fig. 2.
DCs pulsed with gp96-peptide complexes derived from SMMC-7721 cells stimulated autologous T cell proliferation. DCs were incubated with 40 μg/ml gp96-peptide complexes derived from SMMC-7721 for 4 h at 37°C and cocultured with autologous T cells at different ratios for 5 days, and then tritiated thymidine was added and thymidine incorporation was measured 18 h later. 1 × 105 T cells were cocultured with 1 × 104 or 3.3 × 104 autologous DCs, pulsed with gp96-peptide complexes (indicated as gp96 stimulation) or unpulsed (indicated as DC control), or by themselves (indicated as T control) per well. The results are expressed as mean ± SD and each value represents the mean of three replicates
DCs pulsed with gp96-peptide complexes derived from SMMC-7721 cells induce antitumor CTLs specific for SMMC-7721
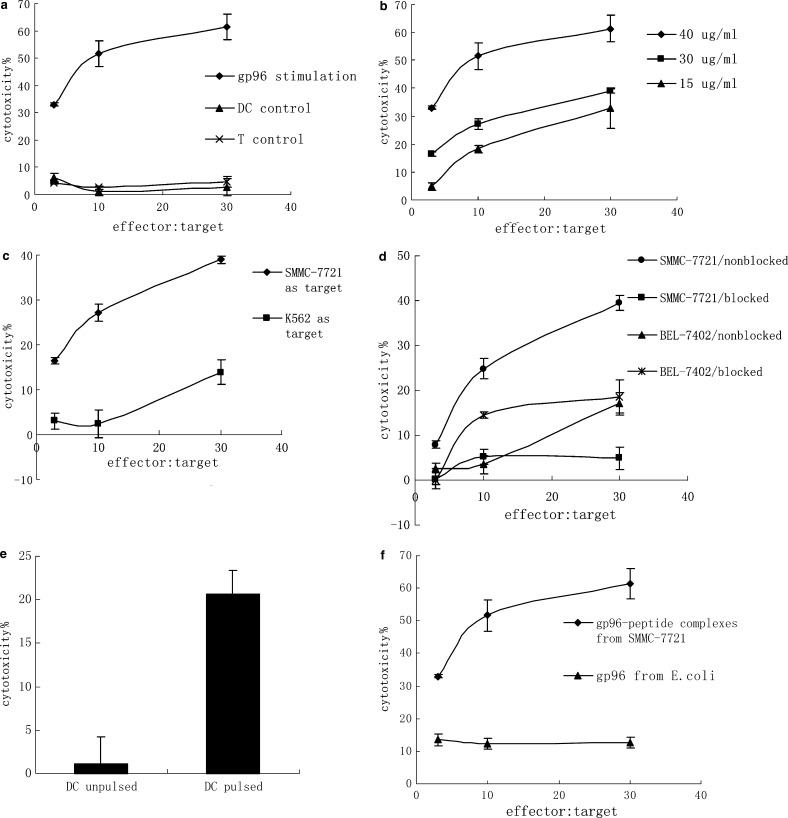
To demonstrate if DCs pulsed with gp96-peptide complexes derived from SMMC-7721 cells could induce specific antitumor CTLs, autologous T cells isolated by nylon wool column were cocultured with pulsed DCs for 7–10 days in the presence of 20 units/ml human IL-2 and then the T cells were isolated for cytotoxicity testing. As a control, the T cells were cultured alone or cocultured with unpulsed DCs. The cytotoxicity results showed that T cells cocultured with DCs pulsed with the gp96-peptide complexes exhibited a high level of lysis against SMMC-7721 cells (Fig. 3a). In contrast, T cells cultured alone or cocultured with unpulsed DCs failed to kill SMMC-7721 cells (Fig. 3a). Also, T cells stimulated by DCs pulsed with higher concentrations of human gp96-peptide complexes generated higher cytotoxicity against SMMC-7721 cells (Fig. 3b). To test if the lysis was due to the presence of NK cells, the NK cell sensitive cell line K562 was used as a target cell. T cells stimulated with DCs pulsed with human gp96-peptide complexes showed very little cytotoxicity against K562; the cytotoxicity was much lower than that against SMMC-7721 (Fig. 3c). The result indicated that it was not NK cells that killed SMMC-7721 cells but rather CTLs.
Fig. 3.
DCs pulsed with gp96-peptide complexes derived from SMMC-7721 cells induced antitumor CTLs specific for SMMC-7721. 2 × 105 DCs, which had been pulsed with gp96-peptide complexes derived from SMMC-7721 or E. coli for 4 h at 37°C, were cocultured with 1 × 106 autologous T cells for 7–10 days in the presence of 20 units/ml human IL-2 in each well of 24-well culture plate. The stimulated T cells were harvested and the cytotoxicity was determined by testing LDH release. a DCs were incubated with 40 μg/ml gp96-peptide complexes and then used as stimulators for autologous T cells, and SMMC-7721 cells were used as target cells. DCs pulsed with human gp96-peptide complexes were indicated as gp96 stimulation, unpulsed DCs indicated as DC control, and T cells cultured by themselves indicated as T control. b DCs were incubated with different concentrations of human gp96-peptide complexes and then used as stimulators for autologous T cells, and SMMC-7721 cells were used as target cells; c DCs were incubated with 30 μg/ml human gp96-peptide complexes and then used as stimulators for autologous T cells, and SMMC-7721 cells or NK sensitive cell line K562 cells were used as target cells; d DCs were incubated with 30 μg/ml human gp96-peptide complexes and then used as stimulators for autologous T cells, and SMMC-7721 cells or another HCC cell line BEL-7402 cells were used as target cells. The target cells were also preincubated with an anti-MHC class I antibody (W6/32; 1:50 dilution) and assayed for lysis, indicated as SMMC-7721/blocked or BEL-7402/blocked, and lysis for the target cells without preincubation with anti-MHC class I antibody were indicated as SMMC-7721/nonblocked or BEL-7402/nonblocked. e DCs were incubated with 30 μg/ml human gp96-peptide complexes and then used as stimulators for autologous T cells, and autologous DCs pulsed with human gp96-peptide complexes or unpulsed DCs were used as target cells. The ratio of effector: target was 10. f DCs were incubated with 40 μg/ml gp96-peptide complexes derived from SMMC-7721 cells or recombinant human gp96 isolated from E. coli and then used as stimulators for autologous T cells, and SMMC-7721 cells were used as target cells. The results are expressed as mean ± SD and each value represents the mean of three replicates
To test if the cytotoxicity was specific to SMMC-7721 and was MHC class I restricted, HCC cell lines SMMC-7721 and BEL-7402 (both of which are MHC class I and human gp96 antigen positive) were used as target cells and were preincubated with anti-MHC class I antibody. As shown in Fig. 3d, the cytotoxicity against SMMC-7721 was much higher than that against BEL-7402. Preincubation of SMMC-7721 cells with anti-MHC class I antibody resulted in the abrogation of tumor cell lysis, however, the anti-MHC class I antibody had little, if any effect on lysis of BEL-7402. The result indicated that the cytotoxicity against SMMC-7721 cells was MHC class I restricted and, therefore, due to CD8+ T cells directed against tumor-specific antigens. In addition, the weak lysis against K562 (Fig. 3c) and BEL-7402 (Fig. 3d) as well as the strong lysis against SMMC-7721 indicated that the specific killing activity of the CTLs was specific to the tumor antigen derived from SMMC-7721.
To exclude the possibility that the SMMC-7721 specific cytotoxicity was due to allogeneic response, autologous DCs pulsed with human gp96-peptide complexes or unpulsed were used as target cells in CTL assay. As shown in Fig. 3e, the CTLs induced by DCs pulsed with human gp96-peptide complexes exhibited significant cytotoxicity against DCs pulsed with gp96-peptide complexes which expressed tumor antigens on their surface, whereas there was quite little cytotoxicity against unpulsed DCs. This result confirmed that the cytotoxicity against SMMC-7721 was a tumor-specific response rather than an allogeneic response.
To further address the specificity of the generated CTLs against SMMC-7721, DCs were pulsed with recombinant human gp96, which did not possess SMMC-7721 tumor-specific antigen, was expressed in E. coli as a soluble protein, and was purified by a set of chromatography as described in Materials and methods. As shown in Fig. 3f, T cells cocultured with these DCs pulsed with recombinant human gp96 exhibited very little cytotoxicity against SMMC-7721 cells. The cytotoxicity was significantly lower than that of T cells stimulated with DCs pulsed with human gp96-peptide complexes derived from SMMC-7721 cells. This result showed that DCs pulsed with recombinant human gp96, which did not possess SMMC-7721 associated antigens, could not induce SMMC-7721 specific CTLs, whereas DCs pulsed with human gp96-peptide complexes which possessed SMMC-7721 tumor-associated antigens could, demonstrating that the generated CTLs were specific to the SMMC-7721 associated antigens.
From the high and specific cytotoxicity of CTLs against SMMC-7721, it can be concluded that the tumor-specific antigens chaperoned by human gp96 have been functionally presented through the interaction of human gp96 with DCs. These findings demonstrate that DCs pulsed with human gp96-peptide complexes are very effective in inducing CTLs specific to SMMC-7721 cells from which the human gp96-peptide complexes are purified.
Gp96-peptide complexes derived from SMMC-7721 cells induce maturation of human DCs
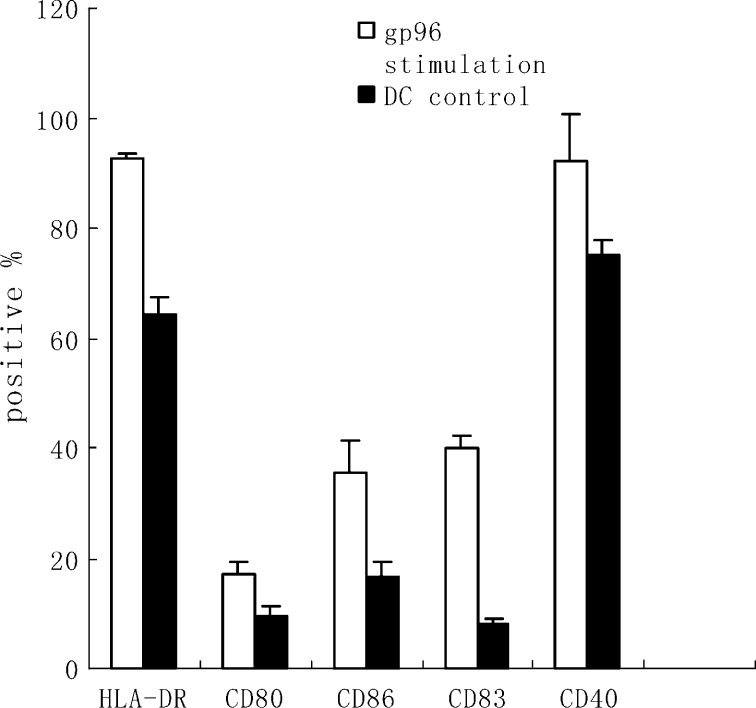
To study the effect of gp96-peptide complexes derived from SMMC-7721 cells on DCs, immature DCs generated by culturing human PBMC in the presence of hGM-CSF and hIL-4 for 6 days, were incubated with human gp96-peptide complexes for 4 h at 37°C and then cultured for 48 h. The expression level of HLA-DR, CD80, CD86, CD83 and CD40 was determined by flow cytometry. As shown in Fig. 4, human gp96-peptide complexes increased expression of HLA-DR, costimulatory molecules CD80 and CD86, and maturation markers CD83 and CD40. This result showed that human gp96-peptide complexes induced maturation of DCs. The maturation effect of human gp96-peptide complexes on DCs suggests that they are strong DC activators.
Fig. 4.
Gp96-peptide complexes derived from SMMC-7721 cells induced maturation of human DCs. DCs were incubated with 40 μg/ml gp96-peptide complexes derived from SMMC-7721 cells for 4 h at 37°C, and cultured for 48 h. The DCs were then harvested and analyzed for expression of the indicated surface molecules using FACS analysis. The percentages shown were positive for the indicated surface markers. The results are expressed as mean ± SD and each value represents the mean of three replicates
Discussion
Cancer has become one of the most common causes of death in industrialized societies [23]. But it is difficult to cure cancer completely using conventional treatment such as surgery, radiation or chemotherapy. New therapies are urgently needed.Immunotherapy, which seeks to eliminate tumor cells by activating the patient’s immune system, is a new and potentially effective approach for treatment of cancer.
The majority of human tumors are antigenic and not immunogenic [27]. The poor immunogenicity of tumors is believed to derive from the ability of tumor cells to escape immune surveillance through different mechanisms such as deleting or down-regulating MHC expression, losing tumor antigens by mutation, not expressing costimulatory molecules, producing immune-inhibitory molecules or deficiency in antigen processing and presentation [17, 27]. During the antitumor immune responses, T cells play a central role. Activating tumor-specific CTLs is the key step in antitumor immunotherapy. T cell activation requires two signals. Signal one is generated when TCR interacts with the MHC-peptide complex and signal two is provided by costimulatory molecules which are expressed by professional APCs. Most tumors do not express costimulatory molecules and thus fail to activate tumor-specific T lymphocytes [13]. The HCC cell line SMMC-7721 does not express costimulatory molecules, such as CD80 or CD86 (data not shown). It is therefore critical to find a way to present HCC antigens together with costimulatory molecules in order to stimulate HCC specific CTLs.
The DCs are potent APCs capable of initiating both primary and secondary immune responses. The potency of DCs derives from their expression of MHC class I, MHC class II, costimulatory and adhesion molecules that cause antigens to be presented efficiently [6, 19]. Tumor immunotherapy using DCs pulsed with tumor antigens has been widely studied in vitro and in vivo. The source of antigen used to load DCs directly affects the efficiency of such tumor vaccines. Because of the instability of tumor cells and the HLA haplotype restriction for certain tumor epitopes, it is ideal to use multiple epitopes as the antigen source for pulsing DCs. This approach can stimulate multiple immune responses against both identified and unidentified tumor-specific antigens.
In the present study, we used HSP, gp96-peptide complexes derived from human HCC cells as the source of antigen to load DCs. Gp96 plays an essential role in protein metabolism and exerts stimulatory activities on innate and adaptive immunity. Studies have shown that vaccination with tumor-derived gp96-peptide complexes induces specific immune responses to the tumors from which the complexes are derived [20, 24, 29, 38, 42]. The ability of gp96 to interact with APCs through specific receptors and to chaperone its bound peptides to be efficiently presented contributes to the high immunogenicity of gp96-peptide complexes [16, 35]. Gp96-peptide complexes are comprised of different tumor antigens, including both identified and unidentified antigens, and can effectively present the tumor peptides together with costimulatory molecules when loaded onto DCs. The roles of gp96 as chaperone and natural adjuvant make gp96-peptide complexes derived from tumor cells a very potent antigen source for loading DCs.
We demonstrated that DCs pulsed with gp96-peptide complexes derived from human HCC cell line SMMC-7721 stimulated significant autologous T cell proliferation, whereas unpulsed DCs had little stimulatory effect because T cells were tolerant toward self-antigens expressed by DCs (Fig. 2). More importantly, we proved both the efficiency and specificity of DCs pulsed with human gp96-peptide complexes for inducing CTLs against tumor cells from which these gp96-peptide complexes were derived (Fig. 3). Our results showed that the cytotoxicity against SMMC-7721 cells was not due to the presence of NK cells but to CTLs, because NK sensitive cell line K562 could not be killed (Fig. 3c). In addition, we tested the phenotype of the cytotoxic cells using T cell lineage marker, CD3, NK cell lineage marker, CD56, NK-T cell lineage marker, CD56+CD3, and monocyte lineage marker, CD14 by flow cytometry (data not shown). The result showed that most of the cytotoxic cells were T cells (89.38%), only a very few were NK cells (4.56%), NK-T cells (4.78%) or other cells (1.28%), and there were even fewer monocytes. Also, we demonstrated that the generated CTLs exhibited specific cytotoxicity toward SMMC-7721 cells but not toward BEL-7402 cells and that the cytotoxicity was MHC class I restricted (Fig. 3d). From the low cytotoxicity to BEL-7402 cells and high cytotoxicity toward SMMC-7721 cells, it can be deduced that the generated CTLs were directed to SMMC-7721 specific antigens but not to their shared antigens. Furthermore, we excluded the possibility that the induced antitumor response was an allogeneic response (Fig. 3e). As previously reported [25, 26, 41], we used autologous DCs pulsed or unpulsed with human gp96-peptide complexes as targets in CTL assay. Our results showed that the generated CTLs exhibited significant cytotoxicity against autologous pulsed DCs, but little lysis against unpulsed DCs, indicating that the CTLs were directed against tumor-specific antigens rather than alloantigens. This result also demonstrated that DCs took up human gp96-peptide complexes, and then processed and presented tumor-specific antigens in MHC class I pathway, making them available for CTL recognition. In addition to showing target cell specificity of the generated CTLs, we also demonstrated the specificity of antigen source for pulsing DCs (Fig. 3f). The result showed that only DCs pulsed with tumor antigen positive human gp96-peptide complexes could induce tumor-cell-specific CTLs. Taken together, our results indicated that the SMMC-7721 specific antigens associated with gp96 are functionally presented through the interaction of human gp96 and DCs. It has been postulated that activation of APCs by gp96 is responsible for the subsequent CTL responses. Our data showed that human gp96-peptide complexes had been taken up by DCs which led to their activation, and the activated DCs then stimulated SMMC-7721 specific CTLs.
Previous studies have shown that HSPs can induce the maturation of DCs [5, 8, 14, 30]. Our data showed that gp96-peptide complexes derived from HCC cell line SMMC-7721 induced maturation of allogeneic DCs, enhancing the expression of HLA class II, costimulatory molecules CD80 and CD86, and maturation makers, CD83 and CD40. Unlike our results, one study reported that mouse gp96-peptide complexes did not induce increased expression of CD40 and CD80 [8]. The different results may be owing to the different sources of gp96-peptide complexes, as they used normal mouse liver, whereas we used human tumor cells for isolating gp96-peptide complexes. The effect of human gp96-peptide complexes on DCs implies that these complexes are capable of directly stimulating DCs to increase their surface expression of HLA and costimulatory molecules. The matured DCs can then present antigens and prime immune responses with high activity.
Our data also showed that human gp96-peptide complexes were capable of inducing CTLs across MHC barriers. MHC matching between SMMC-7721 cells used as source of gp96-peptide complexes and the responding T cells was not required, suggesting that the peptides complexed by human gp96 were not restricted to the MHC haplotype of the donor cells. Such result is consistent with previous reports [12], and provides another example indicating that gp96-peptide complexes can elicit CTLs responses via “cross-priming” [1, 35, 36].
In conclusion, our data shows that gp96-peptide complexes derived from human HCC cell line SMMC-7721, are a very potent antigen source for loading and activating DCs across MHC barriers. The activated DCs are able to efficiently stimulate SMMC-7721 specific CTLs, indicating that DCs, pulsed with gp96-peptide complexes derived from human tumor cells, could be used as potential vaccines in antitumor immunotherapy. Our present findings offer a new kind of antitumor immunotherapeutic strategy—pulsing DCs with gp96-peptide complexes derived from human tumor cells—which is able to activate multiple CTLs efficiently without the need for identifying tumor-specific antigens. In the present study, we used allogeneic rather than autologous tumor cells to isolate gp96-peptide complexes for pulsing DCs derived from healthy donors. We hope to apply this approach to HCC patients in future research.
Acknowledgments
We thank Beijing Red Cross Blood Center for supplying blood samples. We also thank the FACS laboratory of Peking University Health Science Centre for technical support in performing FACS analysis. We appreciate Professor Tian Bo (Institute of Microbiology, Chinese Academy of Sciences) for giving us the plasmid pET30a-gp96.
References
- 1.Arnold D, Faath S, Rammensee HG, Schild H. Cross-priming of minor-histocompatibility antigen specific cytotoxic T cells upon immunization with the heat-shock protein gp96. J Exp Med. 1995;182:885–889. doi: 10.1084/jem.182.3.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arnold-Schild D, Hanau D, Spehner D, Schmid D, Rammensee HG, de la Salle H, Schild H. Cutting edge: receptor-mediated endocytosis of heat shock proteins by professional antigen-presenting cells. J Immunol. 1999;162:3757–3760. [PubMed] [Google Scholar]
- 3.Ashley DM, Faiola B, Nair S, Hale LP, Bigner DD, Gilboa E. Bone marrow-generated dendritic cells pulsed with tumor extracts or tumor RNA induce antitumor immunity against central nervous system tumors. J Exp Med. 1997;186:1177–1182. doi: 10.1084/jem.186.7.1177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baar J. Clinical applications of dendritic cell cancer vaccines. Oncologist. 1999;4:140–144. [PubMed] [Google Scholar]
- 5.Baker-LePain JC, Sarzotti M, Fields TA, Li CY, Nicchitta CV. GRP94(gp96) and GRP94 N-terminal geldanamycin binding domain elicit tissue nonrestricted tumor suppression. J Exp Med. 2002;196:1447–1459. doi: 10.1084/jem.20020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 7.Basu S, Binder RJ, Ramalingam T, Srivastava PK. CD91 is a common receptor for heat shock proteins gp96, hsp90, hsp70, and calreticulin. Immunity. 2001;14:303–313. doi: 10.1016/S1074-7613(01)00111-X. [DOI] [PubMed] [Google Scholar]
- 8.Basu S, Binder RJ, Suto R, Anderson KM, Srivastava PK. Necrotic but not apoptotic cell death releases heat shock proteins, which deliver a partial maturation signal to dendritic cells and activate the NF-kB pathway. Int Immunol. 2000;12:1539–1546. doi: 10.1093/intimm/12.11.1539. [DOI] [PubMed] [Google Scholar]
- 9.Basu S, Srivastava PK. Heat shock proteins: the fountainhead of innate and adaptive immune responses. Cell Stress Chaperones. 2000;5:443–451. doi: 10.1379/1466-1268(2000)005<0443:HSPTFO>2.0.CO;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berwin B, Hart JP, Pizze SV, Nicchitta CV. Cutting edge: CD91-independent cross-presentation of GRP94 (gp96)-associated peptides. J Immunol. 2002;168:4282–4286. doi: 10.4049/jimmunol.168.9.4282. [DOI] [PubMed] [Google Scholar]
- 11.Bodey B, Bodey B, Jr, Siegel SE, Kaiser HE. Failure of cancer vaccines: the significant limitations of this approach to immunotherapy. Anticancer Res. 2000;20:2665–2676. [PubMed] [Google Scholar]
- 12.Castelli C, Ciupitu AM, Rini F, Rivoltini L, Mazzocchi A, Kiessling R, Parmiani G. Human heat shock protein 70 peptide complexes specially activate antimelanoma T cells. Cancer Res. 2001;61:222–227. [PubMed] [Google Scholar]
- 13.Cavallo F, Martin-Fontecha A, Bellone M, Heltai S, Gatti E, Tornaghi P, Freschi M, Forni G, Dellabona P, Casorati G. Co-expression of B7–1 and ICAM-1 on tumors is required for rejection and the establishment of a memory response. Eur J Immunol. 1995;25:1154–1162. doi: 10.1002/eji.1830250504. [DOI] [PubMed] [Google Scholar]
- 14.Cho KB, Palliser D, Guillen E, Wisniewski J, Young RA, Chen JZ, Eisen HN. A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat shock fusion proteins. Immunity. 2000;12:263–272. doi: 10.1016/S1074-7613(00)80179-X. [DOI] [PubMed] [Google Scholar]
- 15.Dhodapkar MV, Bhardwaj N. Active immunization of humans with dendritic cells. J Clin Immunol. 2000;20:167–174. doi: 10.1023/a:1006681312249. [DOI] [PubMed] [Google Scholar]
- 16.Dong R (1989) Establishment of a human hepatocarcinoma cell line SMMC-7721 and initial observations on its biologic characteristics. In: Primary liver cancer. China Academic Publishers, Beijing, Springer, Berlin Heidelburg New York, pp145–153
- 17.Ferrone S, Finerty JF, Jaffee EM, Nabel GJ. How much longer will tumor cells fool the immune system. Immunol Today. 2000;21:70–72. doi: 10.1016/S0167-5699(99)01569-8. [DOI] [PubMed] [Google Scholar]
- 18.Gong JL, Nikrui N, Chen DS, Koido S, Wu ZK, Tanaka Y, Cannistra S, Avigan D, Kufe D. Fusions of human ovarian carcinoma cells with autologous or allogeneic dendritic cells induce antitumor immunity. J Immunol. 2000;165:1705–1711. doi: 10.4049/jimmunol.165.3.1705. [DOI] [PubMed] [Google Scholar]
- 19.Hajek R, Butch AW. Dendritic cell biology and the application of dendritic cells to immunotherapy of multiple myeloma. Med Oncol. 2000;17:2–15. doi: 10.1007/BF02826210. [DOI] [PubMed] [Google Scholar]
- 20.Janetzki S, Blachere NE, Srivastava PK. Generation of tumor-specific cytotoxic T lymphocytes and memory T cells by immunization with tumor-derived heat shock protein gp96. J Immunother. 1998;21:269–276. doi: 10.1097/00002371-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Janetzki S, Palla D, Rosenhauer V, Lochs H, Lewis JJ, Srivastava PK. Immunization of cancer patients with autologous cancer-derived heat shock protein gp96 preparations: a pilot study. Int J Cancer. 2000;88:232–238. doi: 10.1002/1097-0215(20001015)88:2<232::aid-ijc14>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 22.Lindquist S, Craig E. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 23.Liu BB, Ye SL, He P, Liu YK, Tang ZY. MAGE-1 and related MAGE gene expression may be associated with hepatocellular carcinoma. J Cancer Res Clin Oncol. 1999;125:685–689. doi: 10.1007/s004320050334. [DOI] [PubMed] [Google Scholar]
- 24.Mazzaferro V, Coppa J, Carrabba MG, Rivoltini L, Schiavo M, Regalia E, Mariani L, Camerini T, Marchiano A, Andreola S, Camerini R, Corsi M, Lewis JJ, Srivastava PK, Parmiani G. Vaccination with autologous tumor-derived heat-shock protein gp96 after liver resection for metastatic colorectal cancer. Clin Cancer Res. 2003;9:3235–3241. [PubMed] [Google Scholar]
- 25.Murakami M, Gurski KJ, Marincola FM, Ackland J, Steller MA. Induction of specific CD8+ T-lymphocyte responses using a human papillomavirus-16 E6/E7 fusion protein and autologous dendritic cells. Cancer Res. 1999;59(6):1184–1187. [PubMed] [Google Scholar]
- 26.Ota S, Ono T, Morita A, Uenaka A, Harada M, Nakayama E. Cellular processing of a multibranched lysine core with tumor antigen peptides and presentation of peptide epitopes recognized by cytotoxic T lymphocytes on antigen-presenting cells. Cancer Res. 2002;62(5):1471–1476. [PubMed] [Google Scholar]
- 27.Pandey M, Mathew A, Nair MK. Cancer vaccines: a step towards prevention and treatment of cancer. Eur J Surg Oncol. 1999;25:209–214. doi: 10.1053/ejso.1998.0629. [DOI] [PubMed] [Google Scholar]
- 28.Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362:469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- 29.Rivoltini L, Castelli C, Carrabba M, Mazzaferro V, Pilla L, Huber V, Coppa J, Gallino G, Scheibenbogen C, Squarcina P, Cova A, Camerini R, Lewis JJ, Srivastava PK, Parmiani G. Human tumor-derived heat shock protein 96 mediates in vitro activation and in vivo expansion of melanoma- and colon carcinoma specific T cells. J Immunol. 2003;171:3467–3474. doi: 10.4049/jimmunol.171.7.3467. [DOI] [PubMed] [Google Scholar]
- 30.Singh-Jasuja H, Scherer HU, Hilf N, Arnold-Schild D, Rammensee HG, Toes RE, Schild H. The heat shock protein gp96 induces maturation of dendritic cells and down-regulation of its receptor. Eur J Immunol. 2000;30:2211–2215. doi: 10.1002/1521-4141(2000)30:8<2211::AID-IMMU2211>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Singh-Jasuja H, Toes RE, Spee P, Munz C, Hilf N, Schoenberger SP, Ricciardi-Castagnoli P, Neefjes J, Rammensee HG, Arnold-Schild D, Schild H. Cross-presentation of glycoprotein 96-associated antigens on major histocompatibility complex class I molecules requires receptor-mediated endocytosis. J Exp Med. 2000;191:1965–1974. doi: 10.1084/jem.191.11.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Srivastava PK. Purification of heat shock protein-peptide complexes for use in vaccination against cancers and intracellular pathogens. Methods. 1997;12:165–171. doi: 10.1006/meth.1997.0464. [DOI] [PubMed] [Google Scholar]
- 33.Srivastava PK, Amato RJ. Heat shock proteins: the ‘swiss army knife’ vaccines against cancers and infectious agents. Vaccine. 2001;19:2590–2597. doi: 10.1016/S0264-410X(00)00492-8. [DOI] [PubMed] [Google Scholar]
- 34.Srivastava PK, Jaikaria NS. Methods of purification of heat shock protein-peptide complexes for use as vaccines against cancers and infectious diseases. Methods Mol Biol. 2000;156:175–186. doi: 10.1385/1-59259-062-4:175. [DOI] [PubMed] [Google Scholar]
- 35.Suto R, Srivastava PK. A mechanism for the specific immunogenicity of heat shock protein-chaperoned peptides. Science. 1995;269:1585–1588. doi: 10.1126/science.7545313. [DOI] [PubMed] [Google Scholar]
- 36.Suzue K, Zhou XZ, Eisen HN, Yong RA. Heat shock fusion proteins as vehicles for antigen delivery into the major histocompatibility complex class I presentation pathway. Proc Natl Acad Sci. 1997;94:13146–13151. doi: 10.1073/pnas.94.24.13146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Takayama T, Sekine T, Makuuchi M, Yamasaki S, Kosuge T, Yamamoto J, Shimada K, Sakamoto M, Hirohashi S, Ohashi Y, Kakizoe T. Adoptive immunotherapy to lower postsurgical recurrence rates of hepatocellular carcinoma: a randomized trial. Lancet. 2000;356:802–807. doi: 10.1016/S0140-6736(00)02654-4. [DOI] [PubMed] [Google Scholar]
- 38.Tamura Y, Peng P, Liu K, Daou M, Srivastava PK. Immunotherapy of tumors with autologous tumor-derived heat shock protein preparations. Science. 1997;278:117–120. doi: 10.1126/science.278.5335.117. [DOI] [PubMed] [Google Scholar]
- 39.Thurner B, Roder C, Dieckmann D, Heuer M, Kruse M, Glaser A, Keikavoussi P, Kampgen E, Bender A, Schuler G. Generation of large numbers of fully mature and stable dendritic cells from leukapheresis products for clinical application. J Immunol Methods. 1999;223:1–15. doi: 10.1016/S0022-1759(98)00208-7. [DOI] [PubMed] [Google Scholar]
- 40.Vabulas RM, Braedel S, Hilf N, Singh-Jasuja H, Herter S, Ahmad-Nejad P, Kirschning CJ, Costa C, Rammensee HG, Wagner H, Schild H. The endoplasmic reticulum-resident heat shock protein gp96 activates dendritic cells via the Toll-like receptor 2/4 pathway. J Biol Chem. 2002;277:20847–20853. doi: 10.1074/jbc.M200425200. [DOI] [PubMed] [Google Scholar]
- 41.Yang S, Kittlesen D, Slingluff CL, Jr, Vervaert CE, Seigler HF, Darrow TL. Dendritic cells infected with a vaccinia vector carrying the human gp100 gene simultaneously present multiple specificities and elicit high-affinity T cells reactive to multiple epitopes and restricted by HLA-A2 and -A3. J Immunol. 2000;164(8):4204–4211. doi: 10.4049/jimmunol.164.8.4204. [DOI] [PubMed] [Google Scholar]
- 42.Yedavelli Int J Mol Med. 1999;4:243. doi: 10.3892/ijmm.4.3.243. [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Liu DQ, Liu YZ, Wang P, Chen XY. Preliminary study on apoptosis of BEL-7402 cells induced by Chinese herbs for warming yang and dispersing stasis. Zhongguo Zhong Yao Za Zhi/China J Chin Mater Med. 2000;25(7):428–430. [PubMed] [Google Scholar]
- 44.Zitvogel L, Mayordomo JI, Tjandrawan T, Deleo AB, Clarke MR, Lotze MT, Storkus WJ. Therapy of murine tumors with tumor peptide-pulsed dendritic cells: dependence on T cells, B7 costimulation, and T helper cell 1-associated cytokines. J Exp Med. 1996;183:87–97. doi: 10.1084/jem.183.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]