Abstract
Aim and background: CD4+CD25+ cells are described as professional regulatory/suppressor T cells that are crucial for the prevention of spontaneous autoimmune diseases. They play an important role in maintaining a balanced peripheral immune system. On the other hand, it has been suggested that regulatory T cells (Treg) suppress antitumor immune responses after tumor-specific vaccinations. Therefore, we determined the percentage of regulatory T cells in cytokine-induced killer (CIK) cells, an effector cell population with high impact for adoptive immunotherapeutic strategies. Results: CIK cells showed strong induction of CD4+CD25+ cells with high secretion of interleukin 10 (IL-10) after unspecific stimulation of the TCR complex and stimulation with interleukin 2. Depletion of CD25+ cells led to an increase in cytotoxic activity and a reduction of IL-10 release. A more pronounced reversal of suppression could be induced by coculture of CIK cells with dendritic cells (DCs). After coculture of CIK cells with DCs, the number of CD4+CD25+ cells as well as the IL-10 concentration in the supernatant decreased, and the cytotoxic activity against pancreatic carcinoma cells increased. This was shown for cells from healthy donors as well as for cells from patients with pancreatic carcinoma. Conclusion: Our established effector cells possess some regulatory features induced by unspecific TCR-activation that could be prevented by coculture with DCs. CIK cells have desirable properties for immunotherapeutical approaches, especially after coculture with DCs, which could be used additionally for induction of a specific immune response.
Keywords: CIK cells, Dendritic cells, Interleukin 10, Regulatory T cells
Introduction
We developed a protocol to generate large numbers of efficient cytotoxic effector cells [25, 26]. These cytokine-induced killer (CIK) cells are cytotoxic lymphocytes generated from peripheral lymphocytes by a cytokine-cocktail containing anti-CD3 monoclonal antibody. They possess an enhanced cytotoxicity and a higher proliferation rate compared with LAK cells and they are able to lyse tumor cells in a non-major histocompatibility-restricted way [22, 23]. The greater lytic activity of CIK cells is mainly due to the increased proliferation of CD3 and CD56 double positive cells [26]. CIK cells showed antitumor effects against different tumors in vitro as well as in vivo [1, 7, 10, 11, 13, 26, 29, 30]. Despite their high cytotoxic activity against tumor cells, normal human hematopoetic progenitor cells are largely unaffected in a CFU-GM assay [26]. CIK cells were tested in different clinical trials against various tumors (colon carcinoma, astrocytoma, lymphoma, malignant melanoma, and renal cell carcinoma) [4, 28, 32]. All trials showed only mild side effects and produced, as far as it is appropriate to assess this after phase I trials, some clinical responses.
Ongoing studies have provided firm evidence for the existence of a unique CD4+CD25+ population of “professional” regulatory/suppressor T cells, which—in an active and dominant way—prevent both the activation and the effector function of autoreactive T cells that have escaped other mechanisms of tolerance. The elimination or inactivation of these cells resulted in severe autoimmune disease and was also found to enhance immune responses to alloantigens and even to the tumor [5]. CD4+CD25+ regulatory T cells (Treg) constitute a rather homogenous population and are thought to derive from the thymus. They are naturally nonproliferative (i.e., anergic) to stimulation via the TCR but require activation via their TCR to exert suppression [20]. The suppressive function did not appear to be mediated by a soluble factor and seems to be cell-contact dependent. Recent in vivo studies suggest that the function of CD4+CD25+ T cells is crucially dependent on signaling via the cytotoxic T lymphocyte–associated antigen (CTLA)-4/CD152 molecule, which was found to be expressed constitutively on CD4+CD25+ T cells [9]. The number of Treg cells may be elevated in cancer patients [31].
Dendritic cells (DCs) are major antigen-presenting cells that could be used to overcome tumor-related immunosuppression. They play a major role in the immune response to tumor-associated antigens (TAAs), as they capture and process antigens in the periphery, express lymphocyte costimulatory molecules, migrate to lymphoid organs, and secrete mediators to initiate immune responses [8]. Immature DCs are described as inducing Treg cells [14, 19], but mature DCs are well known to activate CIK cells both in an antigen-specific and unspecific way [15–18].
Here, we evaluate the presence of Treg in a CIK population generated from healthy donors as well as from patients with pancreatic carcinoma by determining IL-10 secretion and by testing their cytotoxic activity after coculture with DCs.
Materials and methods
Generation of DCs
Peripheral blood leukocytes were isolated from buffy coats or from blood from patients with pancreatic carcinoma by Ficoll density gradient centrifugation (Lymphoprep; Nycomed, Oslo, Norway). Blood was drawn according to our protocol accepted by the local ethics committee. The cells were allowed to adhere in six-well plates at a density of 5×106 cells/ml for 1 h at 37°C in RPMI 1640 with 10% autologous, heat-inactivated serum. Nonadherent cells were collected for generating CIK cells (see below). Adherent cells were cultured in 2-ml RPMI 1640 with autologous, heat-inactivated serum, 750 U/ml human GM-CSF, and 500 U/ml human IL-4 (Essex Pharma, Nürnberg, Germany) per well for 7 days. Maturation was induced on day +6 by addition of 500 U/ml TNF-α and by CD40-CD40L interactions during coculture [18].
CIK, CD25+, or CD25− cells were harvested on day +8 and cocultured with the DCs at a stimulator to responder ratio of 1:3 for 6 days. This concentration was titrated and found to be optimal.
Generation of CIK cells
CIK cells were generated as previously described. [25, 27] In brief, nonadherent Ficoll-separated human peripheral blood mononuclear cells were prepared and grown in RPMI 1640 medium (Gibco BRL, Berlin, Germany), consisting of 10% fetal calf serum (Gibco BRL, Berlin, Germany), 25-mM HEPES, 100 U/ml penicillin, and 100 μg/ml streptomycin. Human recombinant interferon γ (1,000 U/ml; Roche, Mannheim, Germany) was added on day 0. After 24 h of incubation, 50 ng/ml of an antibody against CD3 (Orthoclone OKT 3; Cilag, Sulzbach, Germany), as well as 100 U/ml interleukin 1β and 300 U/ml interleukin 2 (Roche, Mannheim, Germany) were added. Cells were incubated at 37°C in a humidified atmosphere of 5% CO2 and subcultured every 3 days in IL-2–containing medium at 3×106 cells/ml.
Cell lines
DAN-G (a pancreatic carcinoma cell line) was purchased from DSMZ (Deutsche Sammlung für Mikroorganismen und Zellkultur, Braunschweig, Germany). The cells were maintained in RPMI 1640 supplemented with 10% fetal calf serum (FCS; PAA, Cölbe, Germany), 100 U/ml penicillin, and 100 μg/ml streptomycin (Seromed, Jülich, Germany).
Patients characteristics
We have studied six cases of pancreatic tumors involving six women. The average age at diagnosis was 55.1 years. Five tumors were ductal adenocarcinomas of the head, and one tumor was a papillary mucinous carcinoma. According to the criteria of the International Union against Cancer (UICC), the exocrine pancreatic tumors were classified as one with stage II, four with stage III, and one with stage IV. Informed consent was obtained from each patient to draw 10-ml blood and to publish these experimental data.
Enrichment of CD25+ cells using MACS technique
CD25+ cells were enriched using the MACS technique (Miltenyi, Bergisch Gladbach, Germany) according to the manufacturer’s instructions. In brief, 7-day-old CIK cells were labeled with anti-CD25 beads on ice for 15 min and enriched on a MS MACS column for positive selection. The CD25 negative fraction was eluted after harvest of CD25+ cells.
Measurement of IL-10
Cell culture supernatants were sampled daily and stored at −80 °C. An ELISA development with matched antibody pairs (Biocarta, Hamburg, Germany) was performed according to the manufacturer’s instructions.
Suppression assays
CD25+ cells were enriched from CIK cells by MACS technique on day +13 as described above. CIK cells were mixed with CD25+ cells in different ratios on day +14 immediately before further analysis. To analyze the putative regulatory properties of CD25+ T cells with regard to proliferation, a proliferation assay was used according to the manufacturer’s instructions (EZ4U kit; Biomedica, Vienna, Austria). Cells were seeded out at a density of 2,000 cells per well in triplicate in a 96-well plate. The proliferation rate was determined as the amount of turnover of yellow tetrazolium salt to red formazan. Absorbance at 450 nm with 620 nm as a reference was measured with an ELISA reader; the absorbance of a medium blank was subtracted. The proliferation of CIK cells after coculture with DCs was set to “1.”
Cytotoxicity assay
A standard chromium release assay was used to determine the cytotoxic activity of CIK cells. In brief, DAN-G cells were labeled over 2 h with 100 μCi 51Cr. After three washing steps, 10,000 target cells per well were incubated with CIK cells at different effector to target cell ratios. After 4 h, the supernatant was collected and counts per minute were determined (Packard, Dreieich, Germany). Each experiment was performed in triplicate and the mean value was calculated. Maximum release was obtained by incubating the target cells with 0.1% Igepal (anionic detergent from Sigma). Target cells without effector cells served as a negative control (spontaneous release). The ratio between maximal and spontaneous release was in general >5. Cytotoxicity calculations were performed using the following formula:
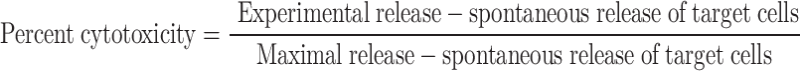 |
Flow cytometry
Cells were incubated with the respective antibodies on ice for 15 min and were then washed with PBS / 1% BSA (phosphate-buffered saline [PBS] from PAA, Cölbe, Germany; bovine serum albumin [BSA] from Sigma). Tricolor flow cytometric analysis was performed using a Coulter Epics XL Cytometer (Coulter-Immunotech, Krefeld, Germany). Data were collected from 30,000 cells and analyzed. CIK cells were phenotyped with the following monoclonal markers: CD4, CD25, and CD152 (all from Pharmingen, Hamburg, Germany); negative controls consisted of CIK cells labeled with mouse IgG.
Statistical analysis
Paired t-test and nonparametrical correlation (Spearman rho) tests were used to determine statistical significance or correlation, respectively (SPSS 11.0). A p value of <0.05 was considered significant.
Results
In vitro generation of DCs and CIK cells
Dendritic cells showed typical cytoplasmic processes and formed characteristic clusters after coculturing with effector cells. The percentage of monocytes (CD14+ cells) declined during culture and DC cultures expressed—besides HLA-DR, CD40, and CD86—the DC-specific markers CMRF-44, CMRF-56, and CD83.
Cytokine-induced killer cells expressed CD3 and the αβ TCRs and were negative for CD16. Apart from CD4+ and CD8+ cells, the population included up to 15% CD3+CD56+ cells at day +14. The percentage of these double-positive cells increased significantly following further cultivation.
Enrichment of CD25+ cells using the MACS technique
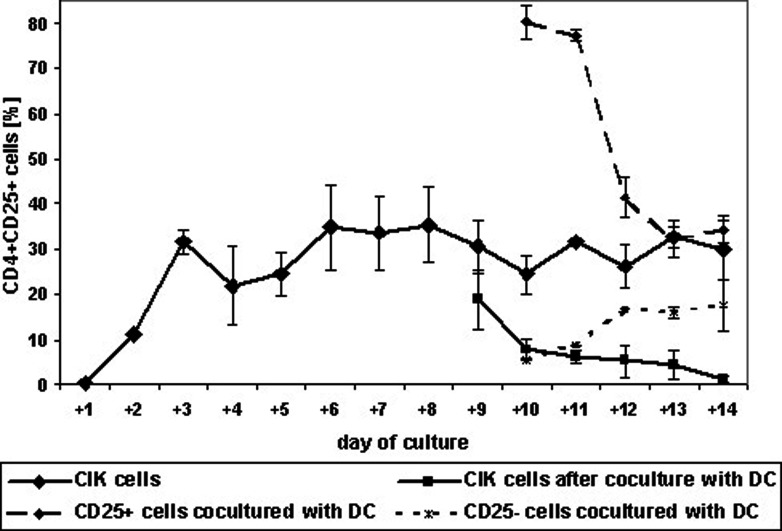
Using MS columns for enrichment of CD25+ cells, we obtained an enriched population with 80.2±3.6% CD4+CD25+ cells and a negative fraction containing 5.4±0.0% CD4+CD25+ cells (Fig. 1).
Fig. 1.
Percentage of regulatory T cells during culture. CIK cells with and without enrichment of CD25+ cells were cultured over 2 weeks. At day +8, they were cocultured with autologous DCs. Expression of CD4 and CD25 was analyzed daily by flow cytometry. Data are shown as mean ± SE from at least three separate experiments
Detection of CD4+CD25+ cells
Cytokine-induced killer cells from healthy donors were stained daily during culture for CD4+CD25+ cells. Only a few Treg cells could be detected on the day of preparation and on day +1 (0.5±0.07%; i.e., 1.6±0.15% of CD4+ cells). After stimulation with anti-CD3 and IL-2 on day +1, the number of Treg cells increased to 11.2±0.3% (40.3±0.85% of CD4+ cells). Following further cultivation, the number continued to increase until a maximum was reached on day +8 (35.5±8.4%, or 74.2±15.7% of CD4+ cells). Between day +8 and day +14, the percentage stayed approximately at 30% (Fig. 1).
Cytokine-induced killer cells after coculture with DCs showed a different pattern. Here, the number of Treg cells decreased during culture. One day after coculture, we already had a significant decrease compared with CIK cells (19.0±1.2% vs 30.4±5.8%; p<0.01). On day +14 of coculture, 1.3±0.9% of CD4+CD25+ cells could be detected, compared with 29.8±6.5% for CIK cells (p<0.001; Fig. 1).
The percentage of Treg cells in MACS-enriched CD25+ cells decreased during coculture too. Starting with a purity of 80%, the number declined during the next days of cultivation to the level of CIK cells (34.1±3.1% on day +14; Fig. 1).
Interestingly, we observed an increase of Treg cells in the CD25− fraction during coculture with DCs. Starting coculture on day +8 with a remaining number of 5.4% CD4+CD25+ cells, we detected 7.6±5.7% Treg cells at day +14. This was more than we detected in cocultured CIK cells.
Determination of IL-10 secretion
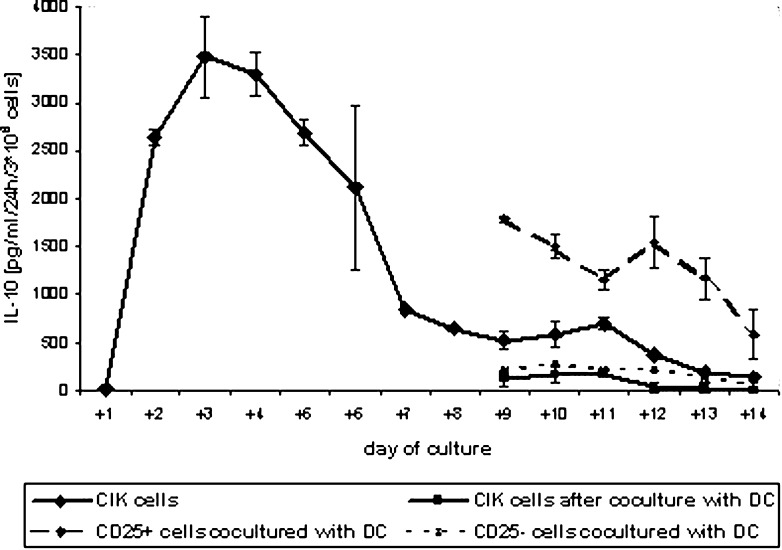
Interleukin 10 concentration in the supernatant was determined each day of culture of CIK cells. During the first 24 h of cultivation, only a small amount of IL-10 could be detected (11.8 ± 11.2 pg/ml/24 h/3×106 cells). Analogous to the number of CD4+CD25+ cells, the amount of IL-10 increased rapidly after stimulation with IL-2 and anti-CD3 on day +1. Twenty-four hours later, 2,634 ± 81.6 pg/ml/24 h/3×106 cells were detectable in the supernatant. After a maximum on day +3 (3,477 ± 415.6 pg/ml/24 h/3×106 cells), the IL-10 concentration decreased and was just 139.6 ± 3.1 pg/ml/24 h/3×106 cells on day +14 (Fig. 2).
Fig. 2.
Secretion of interleukin 10 by CIK cells. To generate CIK cells, PBLs were stimulated with anti-CD3 and IL-2 on day +1 as described in “Materials and methods.” Some CIK cells were purified on a MACS column on day +7 using CD25 beads. On day +8, the cells were cocultured with DCs. Supernatants were stored frozen, and IL-10 concentration was determined by ELISA. Data are shown as mean ± SE from at least three separate experiments
Similar to the number of Treg cells, we observed a decrease in IL-10 secretion for cocultured CIK cells. IL-10 concentration in media of cocultured CIK cells was significantly lower than in CIK cells (p<0.01). On day +14, we observed an IL-10 concentration near the detection level of just 4.8 ± 4.3 pg/ml/24 h/3×106 cells.
Similar results as with the Treg cells were obtained for IL-10 secretion of CD25+/− cells after coculture. CD25+–enriched cells produced high levels of IL-10 (1,789.6 ± 25.5 pg/ml/24 h/3×106 cells between day +8 and day +9), but the amount decreased during coculture (583.8 ± 262.5 pg/ml/24 h/3×106 cells on day +14). The CD25− fraction showed a slow decrease in IL-10 secretion during coculture. Starting with 208.6 ± 8.9 pg/ml/24 h/3×106 cells between day +8 and day +9, we were able to detect 68.2 ± 58.6 pg/ml/24 h/3×106 cells on day +14 (Fig. 2). These levels were almost higher than for CIK cells after coculture.
Cytotoxic activity of CIK cells
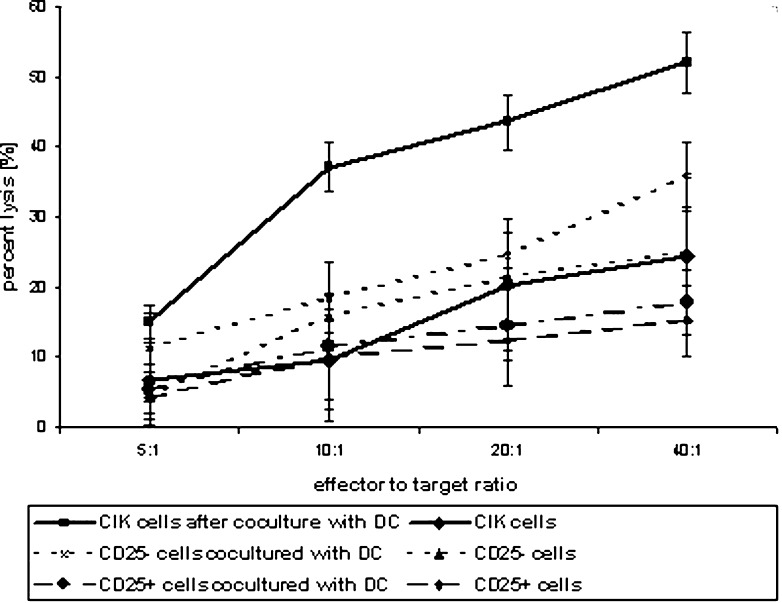
Cytokine-induced killer cells showed moderate cytotoxic activity against DAN-G cells. Cytotoxicity increased during culture (data not shown) and peaked at 24.4±7.0% on day +14 at an effector to target ratio of 40:1. Coculture of CIK cells with DCs resulted in an increase in cytotoxic activity. On day +14, we observed a cell lysis of 52.0±4.3% (all data for an effector to target ratio of 40:1).
With regard to the cytotoxic activity of CD25+/− cells, our results were similar to the other determined parameters. CD25+–enriched cells on their own lysed only a few cells (15.4±5.1%) and coculture could reverse that only marginally (17.9±4.6%). The negative fraction showed moderate activity (24.9±2.8%), which could be enhanced by coculture (35.9±4.9%; Fig. 3).
Fig. 3.
Cytotoxic activity of CIK cells against pancreatic carcinoma cells after coculture with DCs. CIK cells or CD25+/− cells were cocultured with autologous DC cultures from days +8 to +14. Cytotoxic activity at various effector to target cell ratios was measured by chromium-release assay. DAN-G cells were used as target cells. Results show data from at least three separate experiments. Data are shown as mean ± SE
Suppression assays
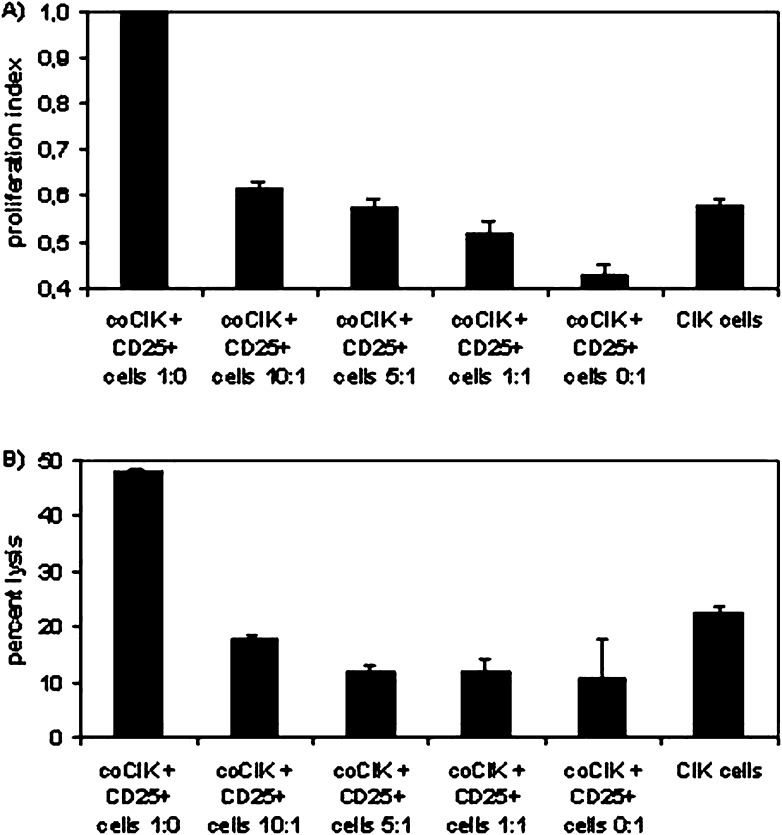
Addition of freshly isolated CD25+ cells from 13-day-old CIK cell culture to CIK cells cocultured with DCs (coCIK) resulted in a decrease of proliferation. This phenomenon was dose-dependent (Fig. 4A). The decrease in proliferation was distinct in pure CD25+ cell cultures. CIK cells without coculture showed, due to the presence of CD25+ cells, a proliferation index similar to coCIK after addition of CD25+ cells in a ratio of 10:1.
Fig. 4A, B.
Suppressive properties of CD25+ cells. PBMCs from healthy donors were used for CIK and DC generation. CD25+ cells were enriched by MACS technique on day +13. CIK cells were mixed with CD25+ cells in different ratios on day +14 immediately before further analysis. A Proliferation index was determined in a nonradioactive assay and calculated by setting the proliferation of CIK cells after coculture with DC cells to “1.” B Cytotoxic activity was measured by chromium-release assay. DAN-G cells were used as target cells. Results show data at an effector to target ratio of 40:1 from at least three separate experiments. Data are shown as mean ± SE
We tested these cultures also in cytotoxicity assays. Again, we found a suppressive effect after addition of CD25+ cells. CoCIK lysed at 48.0±0.7% an effector to target ratio of 40:1; the addition of CD25+ cells diminished this to 10–17% (Fig. 4B).
Correlation between the parameters
We tested the time course of CD4+CD25+ expression, IL-10 secretion, and cytotoxic activity (defined as lytic units) for correlation. Surprisingly, only IL-10 secretion and cytotoxic activity of CIK cells correlated negatively in a significant way (Pearson coefficient −0.601, with p=0.023). Cytotoxic activity and CD4+CD25+ expression correlated positively with a Pearson coefficient of 0.606 and p=0.022. Parameters determined for cocultured CIK cells showed no significant correlation (Table 1).
Table 1.
Testing for correlation of parameters during time course (Spearman rho). For comparing the data, we determined cytotoxic activity of CIK cells on every day of culture and calculated lytic units (data not shown). Lytic units, IL-10 concentration, and percentage of CD4+CD25+ cells during culture were analyzed
| IL-10 secretion | Lytic units | IL-10 secretion after coculture | Lytic units after coculture | |||||
|---|---|---|---|---|---|---|---|---|
| Correlation | P Value | Correlation | P Value | Correlation | P Value | Correlation | P Value | |
| CD4+CD25+ cells | 0.051 | 0.864 | 0.468 | 0.091 | ||||
| IL-10 secretion | −0.525 | 0.044 | ||||||
| CD4+CD25+ cells after coculture | 0.771 | 0.072 | −0.714 | 0.111 | ||||
| IL-10 secretion after coculture | −0.829 | 0.042 | ||||||
Analysis of samples from patients with pancreatic carcinoma
In contrast to what we could do with the peripheral blood lymphocytes from healthy donors, we were able to detect lymphocytes with a regulatory expression profile in PBLs from patients with pancreatic carcinoma without any stimulation. We characterized these cells further by staining with CD152. Analogous to lymphocytes from healthy donors, we observed an increase in the number of Treg cells on day +7 and day +14 of culture with the CIK treatment scheme and a decrease after coculture (Table 2).
Table 2.
Analysis of samples from patients with pancreatic carcinoma for regulatory T cells, IL-10 secretion, and cytotoxic activity. PBLs from patients with pancreatic carcinoma were analyzed for CD4+CD25+CD152+ expression. PBMCs were used for CIK cell and DC generation. CIK ± coculture with DCs were analyzed by flow cytometry and in cytotoxicity assays against DAN-G cells. Sera from patients and media supernatant during culture were analyzed for IL-10 concentration. Results show data for six patients ± SD
| Percentage of CD4+CD25+ cells (CD4+CD25+ from CD4+ cells) | Percentage of CD4+CD25+CD152+ cells (CD4+CD25+CD152+ from CD4+ cells) | IL-10 secretion, pg/ml/3×106 cells/24 h | Percentage lysis of tumor cells, E/T ratio 40:1 | |
|---|---|---|---|---|
| Day 0 | 3.6±1.8 (9.0±5.1) | 1.8±1.1 (4.2±2.2) | 16.3±27.3 | 0.0 |
| Day +7 | 22.3±13.8 (50.0±19.7) | 7.5±3.7 (17.2±9.0) | 641.1±430.5 | 0.0 |
| Day +14 | 30.9±5.7 (58.7±18.8) | 15.7±6.2 (35.9±34.8) | 519.5±232.1 | 7.1±2.1 |
| Day +14 after coculture with DCs | 2.2±1.5 (7.0±2.8) | 1.6±1.0 (2.8±1.9) | 13.6±12.0 | 32.9±8.0 |
Interleukin 10 concentration was determined in sera and in the supernatant of media on day +7 and day +14 of culture of CIK cells. The amount of IL-10 increased during culture. Coculture with DCs decreased the IL-10 concentration in the supernatant. Interestingly, the serum of one patient had a high IL-10 concentration of 82.1 pg/ml to start with, and the concentration increased rapidly after stimulation (1,609 pg/ml on day +7). Furthermore, this patient showed the highest percentage of Treg cells, and her CIK cells had no cytotoxic activity against tumor cells. This patient was the only one who had a papillary mucinous carcinoma; all others had ductal adenocarcinoma of the pancreas.
Cytokine-induced killer cells from patients showed only a little cytotoxic activity against DAN-G cells. Coculture of CIK cells with DCs led to an increase of cytotoxic activity (Table 2).
Discussion
Adoptive transfer of activated T cells circumvents the problem of generating an immune response in an immunosuppressive tumor milieu and could therefore be an option for immunotherapeutical approaches. Different strategies with various types of effector cells were tested in the past in animal and clinical trials. Dudley et al. [6], for example, used highly antigen-specific autologous T cells in patients with metastatic melanoma and obtained very promising results. However, they employed a highly demanding protocol. CIK cells on the other hand are non-MHC restrictive, they have a high cytotoxic capacity as shown in different tumor models, and it is easy to generate them in large numbers [24–26].
Anticancer immunotherapy requires not only potent methods of T-cell activation, but also successful interference with the mechanisms of immune tolerance. Over the last few years, many groups have focused on the immunosuppressive potential of regulatory T cells and shown that Treg cells are potent inhibitors of an antitumor immune response [2, 3, 20]. The increase in Treg cells in the peripheral blood of cancer patients was shown for several tumor entities [12, 31].
Dendritic cells as the most professional antigen-presenting cells were investigated worldwide during the last decade, and at the moment, we can not imagine cancer immunotherapy without them. Using antigen-loaded DCs can direct the immune system to a specific response; but a very important issue is their status of activation, because only fully matured DCs induce activation while immature DCs are responsible for tolerance and induction of Treg cells [14, 19].
Here, we analyzed CIK cells, with and without coculture with DCs, to investigate Treg features. We observed a strong induction of Treg cells after unspecific stimulation of the TCR complex by anti-CD3 antibody. This is not surprising, since the development of Treg cells in the periphery is thought to be triggered by low-affinity antigen or altered TCR signal transduction [3]. These CIK-Treg could be purified by MACS enrichment of CD25+ cells. Coculture of CIK cells, with or without enrichment with DCs, reduces the number and function of Treg cells significantly. Interestingly, CD25− cells acquired increasing regulatory functions following prolonged culture, even in coculture with DCs; a phenomenon that we cannot explain.
Regulatory T cells are described as cells that secrete or induce secretion of IL-10. Therefore, we analyzed the IL-10 pattern during culture. We observed a massive release of IL-10 after unspecific TCR stimulation. Although there was no significant correlation, the time course of IL-10 concentration in medium for CIK cells with or without enrichment and with or without coculture with DCs was generally similar to the time course of CD4+CD25+ cells.
Since we are interested in antitumor effects, we tested the cells for their potential to lyse pancreatic carcinoma cells. Only CIK cells that had been cultured for more than 8 days showed relevant cytotoxic activity (data not shown). As is known, CIK cells cocultured with DCs are more cytotoxic than CIK cells without coculture. The lytic activity of CIK cells correlates significantly with the IL-10 concentration in a negative way. The number of Treg cells correlated significantly with the lytic capacity, but in a positive way. We therefore conclude that IL-10 is a better marker for suppression of cytotoxic activity than CD4+CD25+ expression. One reason for this could be that the unspecific TCR activation induces only a dim expression of CD25. Only cells derived from CD4+CD25+ cells after unspecific T-cell activation are described to express CD25 at a high level, whereas CD4+CD25+ cells derived from CD4+CD25− cells are known to express CD25 at an intermediate level [3, 20]. Since we started with a CD4+CD25− population, the majority of cells had a dim expression, and we were therefore not able to demonstrate a correlation of Treg and cytotoxicity. However, we observed the effect of functional Treg by measuring IL-10 secretion.
After studying the in vitro system, we applied it to cells from patients with pancreatic carcinoma. In general, we observed the same effects regarding number of Treg cells during culture, IL-10 secretion, and cytotoxicity as we did with cells from healthy donors, except for one difference: Contrary to our previous data from healthy donors, we found cells with a regulatory profile in naïve peripheral blood lymphocytes. This is in accordance with data from Liyanage et al. [12] who reported a higher prevalence of Treg cells in patients with pancreatic carcinoma than in normal individuals. However, this is to say that we observed lower levels in healthy donors (1.6% of CD4+ cells) than other groups have reported (between 2.8 and 17.2%). [5, 12, 31]. This may be due to a more or less restrictive setting of regions in flow cytometry, since CD25 in healthy donors has almost a low mean expression and differences between positive and negative populations are not distinct.
To ensure we were detecting Treg cells, on patients’ cells we determined additionally the expression of CD152 (CTLA-4) on CD4+CD25+ cells. On average, 48% of CD4+CD25+ cells showed this constitutively expressed molecule which is well known for its immunosuppressive properties. Blockage of CTLA-4 by antibody has been shown to improve vaccination protocols for cancer [9].
We had one patient with a papillary mucinous cancer of the pancreatic head who showed initially high numbers of CD4+CD25+CD152+ cells (3.9% from PBLs, or 8.4% from CD4+ cells) and an elevated IL-10 level in the serum (82 pg/ml). In this patient, we were not able to reduce Treg or IL-10 secretion, and only a little cytotoxicity could be induced. We therefore hypothesize that patients with massive mucine secretion may have a very poor prognosis similar to mucin-producing colon cancers [31]. Further studies are under way to support this anecdotal experience.
Interactions between DCs and CIK cells have been described to lead to an activation of both populations [18]. Furthermore, Pasare et al. [21] reported that DCs could block suppressor activity of Treg by secretion of IL-6, which was induced by toll-like receptors upon recognition of microbial products. Here, we showed that interactions between CIK cells and DCs are sufficient for blockage of regulatory properties even in the absence of TLR activation. Obviously, antigen-pulsed DCs could be used additionally to induce a specific antitumor response.
In conclusion, we detected some regulatory features in our established effector cells that were induced by unspecific TCR activation. This could be prevented by coculture with DCs. This was shown in five out of six cases for Treg cells found in the blood of patients with pancreatic carcinoma, too. CIK cells have good properties for immunotherapeutical approaches, especially after coculture with DCs.
Acknowledgements
We thank Karin Steybe for excellent technical assistance and Anna Kling for proofreading the manuscript.
References
- 1.Baker Blood. 2001;97:2923. [Google Scholar]
- 2.Barthlott J Exp Med. 2003;197:451. doi: 10.1084/jem.20021387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bluestone Nat Rev Immunol. 2003;3:253. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 4.Chen Ai Zheng. 2002;21:797. [Google Scholar]
- 5.Dieckmann J Exp Med. 2001;193:1303. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudley J Immunother. 2002;25:243. doi: 10.1097/00002371-200205000-00007. [DOI] [Google Scholar]
- 7.Gritzapis Cancer Immunol Immunother. 2002;51:440. doi: 10.1007/s00262-002-0298-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guermonprez Annu Rev Immunol. 2002;20:621. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 9.Hodi Proc Natl Acad Sci U S A. 2003;100:4712. doi: 10.1073/pnas.0830997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hongeng Int J Hematol. 2003;77:175. doi: 10.1007/BF02983217. [DOI] [PubMed] [Google Scholar]
- 11.Linn Br J Haematol. 2002;116:78. doi: 10.1046/j.1365-2141.2002.03247.x. [DOI] [PubMed] [Google Scholar]
- 12.Liyanage J Immunol. 2002;169:2756. doi: 10.4049/jimmunol.169.5.2756. [DOI] [PubMed] [Google Scholar]
- 13.Lu J Immunol. 1994;153:1687. [PubMed] [Google Scholar]
- 14.Mahnke Blood. 2003;23:23. [Google Scholar]
- 15.Märten Cancer Immunol Immunother. 2002;51:25. doi: 10.1007/s00262-001-0251-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Märten Haematoligica. 2001;86:1029. [Google Scholar]
- 17.Märten J Immunother. 2000;23:464. doi: 10.1097/00002371-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 18.Märten J Immunother. 2001;24:502. doi: 10.1097/00002371-200111000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Martin Immunity. 2003;18:155. doi: 10.1016/S1074-7613(02)00503-4. [DOI] [PubMed] [Google Scholar]
- 20.Morse Expert Opin Biol Ther. 2002;2:827. doi: 10.1517/14712598.2.8.827. [DOI] [PubMed] [Google Scholar]
- 21.Pasare Science. 2003;299:1033. doi: 10.1126/science.1078231. [DOI] [PubMed] [Google Scholar]
- 22.Scheffold Bone Marrow Transplant. 1995;15:33. [PubMed] [Google Scholar]
- 23.Schmidt-Wolf Ann Hematol. 1997;74:51. doi: 10.1007/s002770050257. [DOI] [PubMed] [Google Scholar]
- 24.Schmidt-Wolf Br J Cancer. 1999;81:1009. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt-Wolf Br J Haematol. 1994;87:453. doi: 10.1111/j.1365-2141.1994.tb08297.x. [DOI] [PubMed] [Google Scholar]
- 26.Schmidt-Wolf Exp Hematol. 1993;21:1673. [PubMed] [Google Scholar]
- 27.Schmidt-Wolf J Exp Med. 1991;174:139. doi: 10.1084/jem.174.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmidt-Wolf Br J Cancer. 1999;81:1009. doi: 10.1038/sj.bjc.6690800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Verneris J Clin Immunol. 2002;22:131. doi: 10.1023/A:1015415928521. [DOI] [PubMed] [Google Scholar]
- 30.Wang World J Gastroenterol. 2002;8:464. [Google Scholar]
- 31.Wolf Clin Cancer Res. 2003;9:606. [Google Scholar]
- 32.Wood J Neurooncol. 2000;48:113. doi: 10.1023/A:1006456421177. [DOI] [PubMed] [Google Scholar]