Abstract
CD86 and CD80 costimulatory antigens are highly overexpressed on Hodgkin/Reed-Sternberg cells in patients with Hodgkin's disease (HD) and are candidate target antigens for immunotoxins in order to eliminate minimal residual disease. In this study we have evaluated the pharmacokinetics (and immunological) toxicity in rhesus monkeys of immunotoxins consisting of gelonin conjugated to anti-CD86 (αCD86-IT). Both αCD86-IT and αCD80-IT inhibited protein synthesis in B cell lines from rhesus monkeys and inhibited the mixed leukocyte reaction. Reactivity of the αCD86 antibody with rhesus monkey CD86 (RhCD86) was shown by cloning and transfecting RhCD86, which conferred reactivity of the αCD86 antibody used in this study. αCD86-IT was administered as single intravenous bolus injection in four rhesus monkeys, and achieved plasma concentration in 50-fold excess able to eliminate cultured Hodgkin/Reed-Sternberg cells up to 6 h. The animals were capable of generating primary immune responses to both gelonin and murine IgG within 9 days after infusion with αCD86-IT. No evidence could be found of significant (immunological) toxicity. The results of this study show that αCD86-IT can be applied safely in an effective dose.
Keywords: CD86, CD80, Rhesus monkey, Immunotoxin
Introduction
Hodgkin's disease (HD) is a lymphoma characterized by the presence of scarce multinucleated Reed-Sternberg cells and mononucleated Hodgkin's tumor cells which are outnumbered at a ratio of approximately 1:1,000 by surrounding nonmalignant cells [2, 17]. Although most patients can be cured by chemotherapy with or without radiotherapy, about 20% of patients will eventually relapse [3, 6]. This relapse is caused by small numbers of drug-resistant residual tumor cells, remaining after first-line treatment. Elimination of minimal residual disease after initial chemoradiotherapy might improve the outcome in patients with HD, and this may be achieved by eliminating residual Hodgkin/Reed-Sternberg (H/R-S) cells using monoclonal antibodies (MAbs) coupled to protein synthesis inactivating toxins (immunotoxins or "ITs") [11].
Suitable target antigens for IT-therapy must be highly expressed on H/R-S cells but absent on hematopoietic stem cells. Furthermore, they may be expressed only on a small fraction of leukocytes, which can be replenished from the stem cell compartment after elimination by ITs. Candidate antigens to be targeted by ITs in HD include CD15, CD25 (IL-2 receptor), CD30, CD40, CD80 (B7–1), and CD86 (B7–2) [7]. Immunotoxins consisting of anti-CD86 or -CD80 MAbs conjugated to gelonin (αCD86-IT and αCD80-IT) have been tested in vitro, and were shown to be highly efficient in eradicating multiple H/R-S cell lines [15, 22]. CD80 and CD86 are both highly expressed on H/R-S cells, but not on other tissue cells or on CD34+ cells from bone marrow, resting T or B cells from peripheral blood, or epithelial and endothelial cell lines. However, it has been well established that several types of hematopoietic cells express B7-costimatory molecules upon activation, including dendritic cells, macrophages, T and B cells [1, 9, 10]. CD86 and CD80 antigens play an essential role in the induction of primary T-cell responses. Previous experiments have shown that activated PBMCs treated with αCD86-IT lost >95% of stimulatory capacity, suggesting elimination of dendritic cells [15].
In vivo administration of αCD86-IT or αCD80-IT may immunologically compromise treated animals due to elimination of antigen-presenting cells. CD86 is expressed constitutively on dendritic cells and up-regulated prior to CD80 during activation [12]. Treatment with αCD86-IT could prevent the generation of primary immune responses directly by blocking CD86 costimulatory signals and cause depletion of CD86-expressing dendritic cells. Such a compromise of the immune system would exclude the use of αCD86-IT in patients with HD. As any immunological adverse effects in a toxicity study may emerge most clearly upon treatment with αCD86-IT, we focused further studies on this IT. In this study we have assessed the pharmacokinetics (and immunological) toxicity of αCD86-IT in rhesus monkeys.
Materials and methods
Antibodies and reagents
Anti-CD80 (αCD80, clone 5B5, IgG3) and anti-CD86 antibodies (αCD86, clone 1G10, IgG1) were kindly provided by Innogenetics (Ghent, Belgium) and are collectively called αB7-MAbs. The αB7-MAbs were conjugated to gelonin (yielding αCD80-IT and αCD86-IT) as described previously [22]. In combination, αCD80-IT and αCD86-IT are called αB7-IT. The specificity of αCD80-IT and αCD86-IT was proven by their capacity to specifically inhibit protein synthesis of CD80- or CD86-transfected A431 cells, respectively, indicating that after coupling to gelonin, the αCD80 and αCD86 MAbs retained their specific binding activity [16]. Keyhole limpet hemocyanin (KLH) and alkaline-phosphatase-labeled affinity-purified rabbit antimonkey IgG antibodies were purchased from Sigma (St. Louis, MO), complete Freunds' adjuvant (CFA) from Difco laboratories (Detroit, MI), and alkaline-phosphatase-conjugated rat antimouse IgG antibodies from Jackson Immunoresearch Laboratories (West Grove, PA)
Enzyme-linked immunosorbent assay (ELISA)
αCD86-IT, αCD86-MAbs, gelonin, KLH, or B-CII (10 μg/ml) were coated on 96-well microtiterplates in PBS (10 mM sodium phosphate, pH 7.2, 150 mM NaCl) overnight at 4°C. Thereafter, plates were washed three times with PBS + 0.05% Tween-20, and incubated for 1 h at 37°C with PBS + 0.1% BSA. Serially diluted serum of treated and control monkeys were incubated with coated antigen for 2 h at room temperature, then washed three times with PBS + 0.05% Tween-20. Next, alkaline-phosphatase-labeled affinity-purified rabbit antimonkey IgG antibodies in PBS + 0.05% Tween-20 was incubated at room temperature for 2 h followed by three times washing with PBS + 0.05% Tween-20. Color reaction was developed using the para-nitrophenyl phosphate enzyme substrate upon incubation for 30 min at 37°C, absorbance values were read at 405 nm with a microplate reader. Using a sandwich ELISA, concentrations of circulating αCD86-IT were determined. To this end, microtiterplates were coated with (10 μg/ml) antibodies directed against gelonin. Subsequently, diluted monkey sera were added followed by alkaline-phosphatase-conjugated rat antimouse IgG antibodies.
Cell culture and transfectants
A431 cells transfected with human CD80 (A431CD80) or CD86 (A431CD86) were generated, cultured, and propagated as described previously [11]. Rhesus B7–2 was cloned as follows: RNA was extracted from the EBV immortalized rhesus B cell line 3C (BPRC, Rijswijk, The Netherlands) using RNA-SR (Biogenesis, Poole, UK) according to the manufacturer's protocol. RT-PCR for amplification rhesus macaque B7–2 was performed using the Geneamp kit (Perkin Elmer, Branchburg, NY) according to the manufacturer's protocol. Primers used to amplify rhesus B7–2 contain a BAMH1 and a XBA-1 restriction site respectively at the underlined positions, and a GGC or GGG clamp at their 5' position.
Forward primer: GGCGGATCCCAGTGCACTATGAGAC
Reverse primer: GGGTCTAGAGGGCTTTACTCGTTAA
Primer sequences were based on partial matching with human CD86 coding sequences [15]. Amplification was performed in a P9600 thermal cycler (Perkin Elmer) with cycling 1× (105" 95°C), 25× (15 95°C + 30" 55°C + 30" 72°C), 1× (7' 72°C). The resulting PCR product was digested with BAMH1 and XBA-1, ligated into the pcDNA3 vector (In Vitrogen, Leek, The Netherlands), and the mixture was used to transform competent JM109 bacteria. The CD86 inserts of three plasmids were sequenced and found to be identical. A431 cells were transfected by electroporation with pcDNA3 containing rhesus B7–2 coding sequences and grown in medium containing geneticin (Gibco Biocult, Glasgow UK). Resistant cells were expanded and sorted by flowcytometry to select for A431 cells with high expression of B7–2 expression.
Animals
Naïve, captive bred 4–6 kg rhesus monkeys (Macacca mulatta) were obtained, housed, and sedated for routine handling as described previously [14]. Rhesus monkeys containing circulating antibodies directed against αCD86-IT were excluded from this study. Four monkeys were studied, of whom two (designated EEG and DXD) received 0.1-mg/kg and two (designated BJB and EBW) received 0.25-mg/kg αCD86-IT in PBS, given as a single intravenous bolus injection at day 0.
Monitoring effects of αCD86-IT treatment
Blood samples were obtained at day -3, 0, 1, 2, 4, 9, and thereafter once a week until 8 weeks following αCD86-IT treatment. Sera were analyzed for serum electrolytes, liver enzymes, kidney function parameters, and hematological markers by the SSDZ (Delft, the Netherlands). Pharmacokinetics of αCD86-IT was analyzed in the sera taken at multiple time points at day 0 and determined using a sandwich ELISA. The functionality of αCD86-IT upon injection was assessed by measuring protein synthesis inhibition of the CD86 expressing L540 non-Hodgkin's lymphoma cell line upon incubation with sera from the treated monkeys. The titer of rhesus monkey antibodies directed against mouse antibodies (αCD86) or gelonin was also determined by ELISA in which plates were coated with 1G10 or gelonin, respectively. FACS analysis was used to determine the percentage of lymphocytes or monocytes expressing CD80, CD86, or CD40. To examine whether treatment with αCD86-IT induces depletion of dendritic cells, PBMC derived from αCD86-IT–treated monkeys' αCD86-IT were tested for the capacity to induce alloresponses. To this end, responder T cells derived from untreated MHC mismatched rhesus monkeys were mixed with irradiated PBMC derived at different time points from the αCD86-IT–treated monkeys. To assess whether αCD86-IT–treated monkeys can generate a primary immune responses, KLH and denatured collagen type II was injected subcutaneously at day 4, and B-cel responses against KLH were monitored at day -3, +4, and in blood taken once a week for 8 weeks. The appearance of αCD86-IT and presence of CD80 or CD86 expressing cells in lymph-node biopsies was assessed using immunohistochemistry as described previously [8].
Statistical analysis
The Mann Whitney U-test was used to compare percentages of inhibition mediated by αB7-ITs, αB7-MAbs, or gelonin.
Results
In vitro toxicity and B7 costimulatory blocking of anti-B7 immunotoxins
In order to assess whether the antibodies directed against human CD80 and CD86 cross-react with the equivalent expressed in rhesus macaques (RhCD86), B cell lines derived from three different animals were analyzed by flowcytometry. The results show binding of both αCD80 and αCD86 antibodies to RhB cell lines (Fig. 1a). Furthermore, upon coupling of gelonin to αCD80- and αCD86 antibodies, the αCD80-IT and αCD86-IT respectively in concentrations higher that 10-10M were capable of inhibiting protein synthesis on all three of the RhB cell lines (Fig. 1b). To examine whether these results were really due to reactivity with RhCD86 and not some other protein, we cloned the RhCD86 gene. The CD86 sequences were found to be identical to the one published previously [22], except in the leader sequence amino acid positions 2 and 6 were found to be respectively an arginine and threonine instead of a glycine and isoleucine. Sequence comparison indicated that this gene shares 96% homology and the deducted protein sequence 94% homology with human CD86. Transfection of RhCD86 coding sequences in a human CD86-negative A431 cell line conferred αCD86-IT sensitivity (Fig. 2). Furthermore, western blot analysis indicated that the RhCD86 protein expressed in the transfectant has the same molecular weight as that expressed in rhesus B cell lines (data not shown).
Fig. 1a, b.
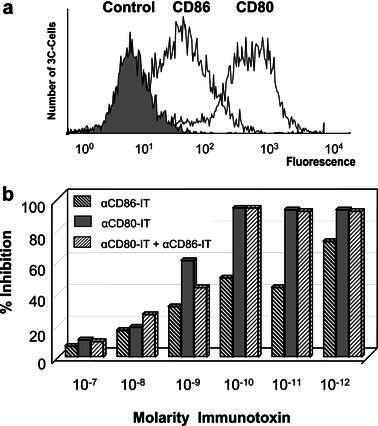
Surface expression of CD80, CD86 on 3C cells. a Rhesus monkey 3C, 2V, 2S, and 3081 EBV transformed B cells were stained with anti-CD80 (clone 5B5), anti-CD86 (clone 1G10), or control IgG, followed by rabbit antihuman FITC-conjugated IgG. Results were analyzed by flow cytometry as indicated in the figure, and shown are the results for cell line 3C. b Cytotoxicity of αCD86-IT and αCD80-IT to rhesus monkey B cell lines. Rhesus monkey 3C cells were incubated with different concentrations αCD86-IT, αCD80-IT, or both, for 72 h and additional 16 h with 3H-leucine. Inhibition of protein synthesis is expressed as percentage of 3H-leucine incorporation by untreated cells. Concentrations refer to the amount of gelonin in the IT used. Gelonin alone did not induce significant inhibition of protein synthesis (not shown). Data represent mean of three independent experiments. Standard deviations were less than 10%
Fig. 2.
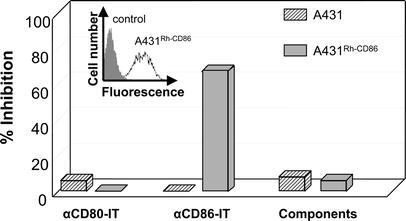
Cytotoxicity of αCD86-IT and αCD80-IT to RhCD86-transfected A431 cells. A431 cells transfected with RhCD86 were stained with anti-CD86 (clone 1G10) or control IgG followed by rabbit antihuman FITC-conjugated IgG. Results were analyzed by flow cytometry as indicated in the inserted figure. Both RhCD86 and mock transfected A431 cells were incubated with 10-8M αCD86-IT, αCD80-IT or unconjugated MAbs plus free gelonin for 72 h and additional 16 h with 3H-leucine. Inhibition of protein synthesis is expressed as percentage of 3H-leucine incorporation by untreated cells. Concentrations refer to the amount of gelonin in the IT used
To examine whether the capacity of αCD80 and αCD86 antibodies to block B7 costimulatory signalling is retained after conjugation to gelonin, both ITs were used in a primary MLC. The results show that αCD86-IT and αCD80-IT could inhibit 80–95% of the MLC responses respectively, whereas αCD86 and αCD80 antibodies induce inhibition ranging from 55–80% and 10–60%, respectively (Fig. 3). Statistical analysis demonstrated that both ITs are significantly more efficient than their unconjugated counterparts in inhibiting proliferative T-cell responses in the MLC (P<0.001).
Fig. 3.
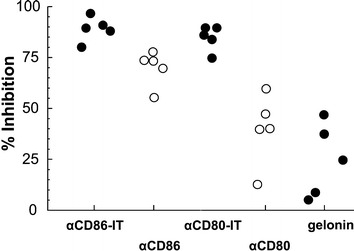
Comparison of ITs, unconjugated MAbs, and gelonin in the MLR. Mixed leukocyte reactions were performed as described in "Materials and methods," in the presence of 10-9M αCD86-IT, αCD80-IT, αCD86, αCD80, or gelonin. At day 5, 1-μCi 3Hthymidine was added to each well and harvested 20 h thereafter. Open circles represent unconjugated MAbs, solid circles ITs and shaded circles gelonin. The results are expressed in percentage inhibition of 3Hthymidine incorporation compared with untreated control MLR values
Pharmacokinetics of αCD86-IT
To assess the pharmacokinetics and toxicity, αCD86-IT was intraveneously administered to four rhesus macaque monkeys. Infusion of 0.1-mg/kg (monkeys DXD and EEG) or 0.25-mg/kg (monkeys BJB and EBW) αCD86-IT resulted within 15 min in peak plasma concentrations of 0.06–0.1×10-7M (0.1 mg/kg bolus) and 0.6–1.0×1.0-7M (0.25 mg/kg bolus), respectively (Fig. 4a and 4b). After 2 h, the average concentrations in plasma were reduced to 0.02–0.04×10-7M (0.1 mg/kg bolus) and 0.1–0.3×1.0-7M (0.25 mg/kg bolus), which is less than 50% of peak plasma levels. To assess whether the immunotoxin was functionally present within the circulation, serum of infused monkeys taken at multiple time points was tested for its capacity to inhibit protein synthesis in CD86-expressing RhB cell lines (Fig. 5a shows dilution 1:50, and Fig. 5b shows dilution 1:10). The results show that αCD86-IT is present within the circulation in effective concentrations, resulting in 90% inhibition of protein synthesis, up until 6 h after infusion. Addition of unconjugated αCD86 to sera from monkeys treated with αCD86-IT prevented the inhibition of protein synthesis in CD86-expressing RhB cell lines (data not shown). In control experiments, cytotoxicity of αCD86-IT was not affected by addition of control sera from untreated monkeys (data not shown).
Fig. 4a, b.
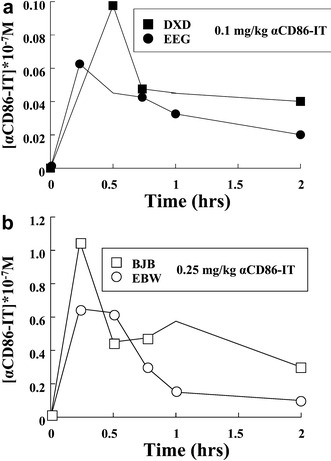
Pharmacokinetics of αCD86-IT intravenously applied in rhesus monkeys. Concentrations of αCD86-IT were measured by ELISA as described in "Materials and methods." a Two monkeys were treated with 0.1-mg/kg αCD86-IT (Fig. 3a). b Two monkeys were treated with 0.25-mg/kg αCD86-IT (Fig. 3b)
Fig. 5.
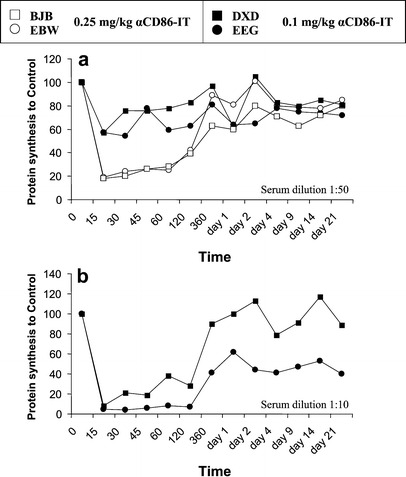
Inhibition of protein synthesis by serum of αCD86-IT–treated rhesus monkeys. Sera from rhesus monkeys taken at the indicated times were added to L540 cells for 72 h and additional 16 h with 3H-leucine. Inhibition of protein synthesis is expressed as percentage of 3H-leucine incorporation by untreated cells. a Sera from all monkeys were analyzed at dilutions 1:50 (Fig. 4a). b From the two monkeys treated with 0.1-mg/kg αCD86-IT, sera were also analyzed at 1:10 (Fig. 4b)
Immunological responses and in vivo toxicity following administration of αCD86-IT
None of the four monkeys showed any signs of toxic complications or infections. With the exception of the liver enzymes SGPT and SGOT (all animals showed an anesthesia-associated increase from day 1–4 and day 1, respectively), significant changes in serum electrolytes or immunoglobulin concentrations were not observed. The two animals treated with 0.1 mg/kg αCD86-IT showed an increase in neutrophil count from day 9–21 post infusion. A clear increase in percentage of monocytes expressing CD40 could be observed in the two animals treated with 0.25-mg/kg αCD86-IT (Fig. 6), whereas the percentage of CD80- or CD86-expressing monocytes remained unchanged. No significant changes in the percentage of CD80-, CD86-, or CD40-expressing lymphocytes were observed in any treated monkey. Immunohistochemical examination of lymph node biopsies in the treated animals showed no reduction in CD86 or CD80 expression, and no histological effect could be seen of αCD86-IT treatment on cellular composition or expression of membrane-bound antigens in lymph nodes.
Fig. 6.
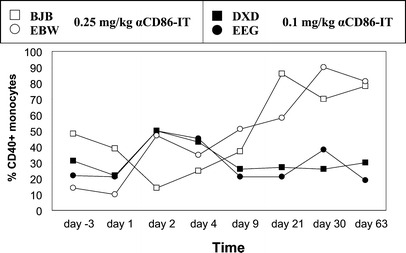
Expression of CD40 on monocytes of αCD86-IT–treated rhesus monkeys. PBMCs from rhesus monkeys were stained with anti-CD14 conjugated with biotin and anti-CD40 conjugated with PE, followed by streptavidin-FITC. Depicted is the percentage of CD40+ cells with in CD14 gate, as analyzed by flow cytometry
To examine whether treatment with αCD86-IT induces depletion of dendritic cells, PBMC derived from αCD86-IT–treated monkeys were tested for the capacity to induce alloresponses as measured in the MLC. The allogeneic T-cell stimulatory capacity of PBMC from the αCD86-IT–treated monkeys remained unchanged throughout this study, except on day 1 when a 70% decrease in stimulatory capacity was observed in the monkeys treated with 0.25-mg/kg αCD86-IT. Proliferative responses of PHA-stimulated T cells taken at different times after αCD86-IT T cells did not change significantly thoughout the study. Specific monkey antibodies towards mouse IgG (MAMA) appeared, with comparable kinetics, in the circulation of all four animals at day 9 after αCD86-IT infusion. In the animals treated with 0.1-mg/kg αCD86-IT antibodies against the toxin moiety gelonin (MATA) appeared at day 9 post infusion, whereas anti-gelonin antibodies in the animals treated with 0.25 mg/kg appeared at day 14. These MAMA and MATA antibodies were capable of inhibiting the cytotoxic potency of αCD86-IT-to-CD86–expressing L540 cells (data not shown).
Discussion
CD86 is a candidate target antigen when residual H/R-S cells are to be eradicated in patients with HD treated with chemoradiotherapy. Previous studies have shown the efficacy of αCD86-IT in eradicating H/R-S cells, but have also implicated the susceptibility of activated dendritic cells to αCD86-IT [12, 22]. The anti-CD86 MAb used in this study was shown to cross-react with the rhesus macaque equivalent of human CD86. Upon infusion, concentrations of circulating αCD86-IT were sufficient for up to 6 h to efficiently inhibit protein synthesis in CD86-expressing cells. The animals were capable of generating primary immune responses to both gelonin and murine IgG, showing that treatment with αCD86-IT did not immunocompromise the animals. Overall the immunological toxicity was considerably less than expected based on our previous in vitro studies. No significant side effects were observed, including absence of changes in liver enzyme concentration, liver function tests, serum electrolytes, or renal functional tests.
The efficacy and in vivo applicability of an IT depends on both the antibody and toxin component. The toxin moiety in αΒ7-IT, gelonin, is a single-chain 30 kDa type I plant protein with 32% homology to ricin A [13]. Gelonin has no defined cell-binding component and is not toxic to mitogen-activated lymphocytes up to 10-6 M. Both antihuman-CD86 and -CD80 antibodies showed reactivity with B cell lines from rhesus macaque monkeys and, as expected, both αCD86-IT and αCD80-IT could inhibit protein synthesis of these cell lines. As the RhCD86 protein is known to share 94% amino acid homology with human CD86 in the IgV region and 95% in the IgC region [21, 23], it could not be safely assumed that the αCD86 antibody used, actually bound to RhCD86. Cloning and sequencing of RhCD86 revealed differences in the leader sequence, known to occur between monkeys from the same species but from different breeding colonies [23]. Transfection of the RhCD86 coding sequences in a human CD86− human cell line conferred αCD86-IT sensitivity, thus proving interspecies cross-reactivity of the αCD86 antibody used in this study.
When comparing αCD86-IT and αCD80-IT with their unconjugated counterparts in MLC cultures, it was found that the ITs were more efficient in inhibiting MLC responses than the MAbs used. This suggests that during a primary MLC, αCD86-IT and αCD80-IT not only block B7-CD28 interaction but also eliminate stimulatory dendritic cells. Remarkably however, αCD80-IT was much more efficient in inhibiting rhesus macaque MLC responses (70–90%) than human MLC responses (0–55%) [15]. Although the reason for this difference is unknown to us, it may be related to the reported expression of CD80 on circulating T cells in rhesus macaques whereas it is absent on human circulating T cells [20]. Inhibition caused by αCD86-IT appeared comparable between rhesus macaque and human MLC responses [15].
Upon infusion, the levels of circulating αCD86-IT were in ranges reported previously to be sufficient for eliminating CD86-expressing cultured H/R-S cells and inhibiting of primary T-cell responses in vitro [15, 22]. The disulfide-linked toxin-antibody conjugate was stable in serum of macaque monkeys, whereas the cytotoxic potential remained largely unaffected. Sera taken in the first 6 h after infusion, and diluted 10–50-fold, could inhibit 90% protein synthesis of CD86-expressing RhB cell lines. As Hodgkin's lymphomas from patients with HD are well vascularized, it can be envisaged that the concentrations reported in this study are sufficient to eradicate H/R-S cells constitutively expressing CD86. Considering the human situation, in vitro studies showed that preincubation of PBMCs with αCD86-IT did not eliminate their capacity to stimulate alloreactive T cells [15], indicating that circulating blood DC do not constitutively express significant amounts of CD86/CD80 antigens [12]. In line with these findings, PBMCs taken from treated monkeys during peak concentration of αCD86-IT and thereafter, showed no diminishment of their stimulatory capacity.
αCD86-IT–treated monkeys could generate primary immune responses resulting in monkey antibodies towards mouse IgG (MAMA) production in all monkeys starting at day 9 post infusion, in line with previous studies [14]. This indicates that the immune system is not compromised upon infusion with αCD86-IT. The monkeys also formed antibodies against gelonin (MATA; monkey antitoxin antibodies), although they were found at a later time in the two animals treated with 0.25-mg/kg than in the two treated with 0.1-mg/kg αCD86-IT. Previous studies have demonstrated that even when murine IgG is administered continuously, the formation of MAMA results in a rapid decrease of murine IgG concentration [14]. It has also been shown that prolonged administration of unconjugated αCD86 or αCD80 antibodies results in MAMA formation starting at day 12 and 10, respectively, whereas the combined continuous administration induced a delay up to day 20 in MAMA formation. The development of neutralizing antibodies clearly limits the number of courses which can be given with αCD86-IT. Antibody formation against the antibody part might be reduced by the use of recombinant human antibodies derived from phage-displayed antibody fragments [8].
The tolerability and use of immunotoxins directed against CD30 and CD25 (IL-2R) has been evaluated in patients with refractory Hodgkin's lymphoma [4, 5, 18, 19]. Treatment of in total 12 patients with immunotoxins against CD30, composed of Saporin-S6 coupled to the Ber-H2 MAb, resulted in four partial remissions, and three minor responses [4, 5]. In a phase I trial, patients with relapsed CD30+ lymphoma were treated using anti-CD30 MAbs coupled to deglycosylated ricinA-chain. Fifteen patients were evaluable of which one achieved a partial remission, one a minor response, and two stable diseases [18]. In another study, 17 of 18 patients were evaluable for clinical response upon treatment with an anti-CD25 immunotoxin, composed of deglycosylated ricin A-chain coupled to the MAb RFT5 [19]. Clinical reponses included two partial remissions, one minor response, and five patients with stable disease. In all studies, the major dose-limiting adverse effects were related to the vascular leak syndrome.
In summary, αCD86-IT can be given intravenously to rhesus monkeys with very limited toxicity at dosages by where CD86-expressing cells can be eliminated. These results warrant further dose escalation in this animal model, and testing whether αCD86-IT or αCD80-IT can eradicate H/R-S cells in SCID-mice xenografted with human H/R-S cell lines.
Acknowledgements
We thank Dr Louis Boon for his critical reading of this manuscript.
References
- 1.Azuma Nature. 1993;366:76. doi: 10.1038/366076a0. [DOI] [PubMed] [Google Scholar]
- 2.Cossman Lab Invest. 1998;78:229. [PubMed] [Google Scholar]
- 3.DeVita N Engl J Med. 1993;328:560. doi: 10.1056/NEJM199302253280808. [DOI] [PubMed] [Google Scholar]
- 4.Falini Lancet. 1992;339:1195. doi: 10.1016/0140-6736(92)91135-u. [DOI] [PubMed] [Google Scholar]
- 5.Falini Cancer Surv. 1997;30:295. [PubMed] [Google Scholar]
- 6.Ferme Blood. 2000;95:2246. [PubMed] [Google Scholar]
- 7.Gruss Baillieres Clin Haematol. 1996;9:417. doi: 10.1016/s0950-3536(96)80019-9. [DOI] [PubMed] [Google Scholar]
- 8.Huls Nat Biotechnol. 1999;17:276. doi: 10.1038/7023. [DOI] [PubMed] [Google Scholar]
- 9.Linsley Science. 1992;257:792. doi: 10.1126/science.1496399. [DOI] [PubMed] [Google Scholar]
- 10.Lokshin Int Immunol. 2002;14:1027. doi: 10.1093/intimm/dxf073. [DOI] [PubMed] [Google Scholar]
- 11.Matthey Int J Mol Med. 2000;6:509. doi: 10.3892/ijmm.6.5.509. [DOI] [PubMed] [Google Scholar]
- 12.McLellan Eur J Immunol. 1995;25:2064. doi: 10.1002/eji.1830250739. [DOI] [PubMed] [Google Scholar]
- 13.Mujoo Cancer Immunol Immunother. 1995;40:339. doi: 10.1007/s002620050183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ossevoort Transplant Proc. 1998;30:2165. doi: 10.1016/s0041-1345(98)00576-4. [DOI] [PubMed] [Google Scholar]
- 15.Otten Tissue Antigens. 1998;52:270. doi: 10.1111/j.1399-0039.1998.tb03042.x. [DOI] [PubMed] [Google Scholar]
- 16.Otten Transplant Proc. 1997;29:1034. doi: 10.1016/s0041-1345(96)00357-0. [DOI] [PubMed] [Google Scholar]
- 17.Poppema Am J Pathol. 1989;135:351. [PMC free article] [PubMed] [Google Scholar]
- 18.Schnell Clin Cancer Res. 2002;8:1779. [Google Scholar]
- 19.Schnell Leukemia. 2000;14:129. doi: 10.1038/sj.leu.2401626. [DOI] [PubMed] [Google Scholar]
- 20.Sopper Cytometry. 1997;29:351. doi: 10.1002/(SICI)1097-0320(19971201)29:4<351::AID-CYTO12>3.3.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 21.Villinger Immunogenetics. 2001;53:315. doi: 10.1007/s002510100322. [DOI] [PubMed] [Google Scholar]
- 22.Vooijs Br J Cancer. 1997;76:1163. doi: 10.1038/bjc.1997.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Cell Immunol. 1997;177:9. doi: 10.1006/cimm.1997.1098. [DOI] [PubMed] [Google Scholar]


