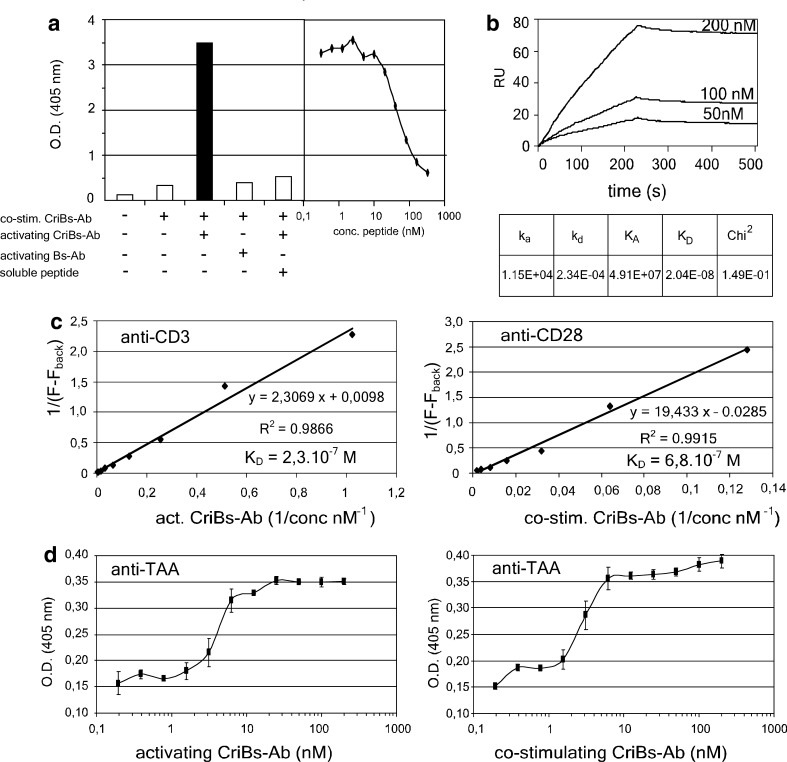
Fig. 4.
Characterization of the CriBs-Abs. a Demonstration of the cross-interaction in an ELISA experiment. An ELISA plate was coated with 25 nM of the P-containing CriBs-Ab and incubated with 50 nM anti-P-containing CriBs-Ab. Mutual binding of the two antibodies was then detected by the elevated trapping of hPLAP activity. As a control, the anti-P CriBs-Ab was substituted with the corresponding Bs-Ab lacking the anti-P moiety. An additional control was performed by adding a dilution series of soluble blocking P-peptide to the anti-P containing CriBs-Ab. b Biologic interaction analysis of the cross-interaction using SPR on a BIAcore 2000: purified co-stimulating CriBs-Ab was coupled to a CM5 chip and increasing concentrations of purified activating CriBs-Ab were used as an analyte. c Determination of the anti-CD3 and the anti-CD28 affinities in the CriBs-Abs by a flow-cytometry-based assay. T-HA cells were incubated with various dilutions of purified activating (anti-CD3) or co-stimulating (anti-CD28) CriBs-Ab (1–500 nM), followed by incubation with 10 μg/ml FITC-conjugated goat anti-mouse Fab. KD is determined by the Lineweaver-Burk method. d The anti-hPLAP affinities of both CriBs-Abs were compared in ELISA. A plate was coated with 10 μg/ml hPLAP and subsequently incubated with a serial dilution (starting at 200 nM) of the activating CriBs-Ab or the co-stimulating CriBs-Ab. The bound CriBs-Abs were detected with an anti-His tag Ab, conjugated to HRP. All experiment were performed in triplicate