Abstract
Background
Prospective randomized trials in severely burned children have shown the positive effects of oxandrolone (OX), beta blockers (BB) and a combination of the two (BBOX) on hypermetabolism, catabolism and hyperinflammation short- and long-term post-burn. Although data on severely burned adults are lacking in comparison, BB, OX and BBOX appear to be commonly employed in this patient population. In this study, we perform a secondary analysis of an international prospective randomized trial dataset to provide descriptive evidence regarding the current utilization patterns and potential treatment effects of OX, BB and BBOX.
Methods
The RE-ENERGIZE (RandomizEd Trial of ENtERal Glutamine to minimIZE Thermal Injury, NCT00985205) trial included 1200 adult patients with severe burns. We stratified patients according to their receipt of OX, BB, BBOX or none of these drugs (None) during acute hospitalization. Descriptive statistics describe the details of drug therapy and unadjusted analyses identify predisposing factors for drug use per group. Association between OX, BB and BBOX and clinical outcomes such as time to discharge alive and 6-month mortality were modeled using adjusted multivariable Cox regressions.
Results
More than half of all patients in the trial received either OX (n = 138), BB (n = 293) or BBOX (n = 282), as opposed to None (n = 487, 40.6%). Per study site and geographical region, use of OX, BB and BBOX was highly variable. Predisposing factors for the use of OX, BB and BBOX included larger total body surface area (TBSA) burned, higher acute physiology and chronic health evaluation (APACHE) II scores on admission and younger patient age. After adjustment for multiple covariates, the use of OX was associated with a longer time to discharge alive [hazard ratio (HR) 0.62, confidence interval (CI) (0.47–0.82) per 100% increase, p = 0.001]. A higher proportion of days on BB was associated with lower in-hospital-mortality (HR: 0.5, CI 0.28–0.87, p = 0.015) and 6-month mortality (HR: 0.44, CI 0.24–0.82, p = 0.01).
Conclusions
The use of OX, BB and BBOX is common within the adult burn patient population, with its use varying considerably across sites worldwide. Our findings found mixed associations between outcomes and the use of BB and OX in adult burn patients, with lower acute and 6-month-mortality with BB and longer times to discharge with OX. Further research into these pharmacological modulators of the pathophysiological response to severe burn injury is indicated.
Keywords: Burns, Critical illness, Hypermetabolism, Oxandrolone, Beta blocker
Highlights.
The use of OX, BB and BBOX is common within the adult burn patient population, with its use varying considerably across sites worldwide.
By analyzing the largest available patient cohort of severely burned adults to date, we give insights into the current clinical use and significance of OX, BB and BBOX in international burn centers.
The findings show mixed associations between outcomes and the use of BB and OX in adult burn patients, with lower acute and 6-month-mortality with BB and longer times to discharge with OX.
BB and OX may be able to modulate the pathophysiological response to burn injury in severely burned adults, with significant benefits for patient outcomes.
Background
Severe burns are among the most devastating forms of trauma and predominantly affect younger adults in low- and middle-income countries [1]. Burns encompassing more than one-fifth of the total body surface area (TBSA) are known to cause a severe pathophysiological stress responses, which can impact multiple organ systems, over a protracted course if the patient recovers from their acute injuries. Acute and long-term systemic perturbations include catabolic hypermetabolism with increased resting energy expenditure (REE) and severe muscle wasting [2], hyperinflammation [3], impaired immune function [4] as well as cardiac dysfunction [5, 6].
Pharmacotherapy aids recovery and improves clinical outcomes following a severe burn by attenuating the hypermetabolic state through several pathways. Beta blockers (BB), such as propranolol, provide systemic blockade of beta adrenergic stimulation, thereby opposing the hypercatabolic pathways, and have been shown to improve clinical outcomes in severely burned children [7]. It has also been shown in randomized controlled trials (RCT), that administration of BB, either during the acute [8, 9] phase of a burn or for up to 1 year later [10], exert numerous positive effects on short- and long-term outcomes in children. Recent studies have elucidated some of the pathways by which BB improve outcomes in burn patients, such as reducing cardiac work, [11] REE [8] and accumulation of central fat [10] and increasing the efficiency of muscular protein synthesis. Two considerably smaller RCTs in severely burned adult patients showed signs of faster donor-site wound healing and diminished perioperative blood loss [11], as well as decreased healing time and hospitalization, without assessing metabolic, inflammatory or organ-specific parameters [12].
In addition to BB, anabolic steroids have been used to reduce the severe catabolism, dysfunctional net protein turnover and marked reduction in lean body mass (LBM) associated with post-burn hypermetabolism in severely burned children. A number of positive effects were noted both acutely as well as after year-long administration of the non-virilizing anabolic steroid oxandrolone (OX) [13]. Results of these randomized clinical trials in pediatric burn patients suggest that the administration of OX reduces cardiac work as well as increasing bone mineral content, LBM and muscle strength, and might shorten hospitalization [14–17]. Again, fewer and smaller (albeit one multicentric) RCTs in severely burned adults were able to demonstrate shorter hospitalization [18] and improved anabolic protein kinetics [19].
Given these benefits in post-burn hypermetabolism, the combination of BB and OX administration was studied in severely burned children and yielded promising results regarding short- and long-term body composition, muscle strength, protein turnover [20], improvements in overall self-reported physical function, as well as subjective and objective measurements of scarring [21]. Nonetheless, prospective evidence for the benefit of these agents, alone and particularly in combination, in severely burned adults remains lacking, and it is presently unknown how often either, or both, of these agents are used in adult burn patients across the world.
The RE-ENERGIZE (RandomizEd Trial of ENtERal Glutamine to minimIZE Thermal Injury, NCT00985205) trial, a phase 3 study which prospectively included 1200 severely burned adults, did not find any benefit in supplementing high-dose glutamine to severely burned adults [22, 23]. This study found no detectable differences in primary outcomes (discharge alive from hospital), as well as secondary (acute and 6-month mortality), and tertiary outcome parameters (bacteremia incidence, urea, creatinine, bilirubin, glucose levels, no difference in cardiac, gastrointestinal, hepatobiliary, nervous system, renal, respiratory, skin and subcutaneous tissue disorders related to exposition to either treatment arm). Nonetheless, the RE-ENERGIZE dataset represents a large, multi-center, multi-national population, which was used to perform a cross-sectional analysis of usage of BB, OX or the combination of the two (BBOX) to provide novel insights into the current usage of these agents in severely burned adults.
Therefore, the purpose of this study was to assess the current status quo of BB and OX use across international burn centers, assess details of drug administration and identify predisposing factors within the severely burned patient population for use of either BB, OX, BBOX or none (None). Further, we aimed to analyze key clinical outcomes associated with administration of BB, OX, BBOX or None in this unique global study population of adults with severe burns.
Methods
As explicated in the previous publications, patients with burns affecting >20% TBSA (18–39 years of age), 15% TBSA with concomitant inhalation injury (18–39 years of age) or 10% TBSA (>60 years of age) were randomly allocated to treatment with either 0.5 g of enteral glutamine per kg of bodyweight per day or placebo during their intensive care unit (ICU) stay [23, 24]. All other clinical decisions, including use of BB, OX, BBOX or None were left to the discretion of the clinical team.
The trial randomized 1209 severely burned adults. A total of 9 patients died, withdrew consent or were discharged before receiving the first dose of study medication, which resulted in an intention-to-treat population of 1200 patients. Data collected included patient demographics [age, sex, ethnicity, body mass index (BMI)], injury characteristics [cause of burn: scald, fire, chemical, other; TBSA affected, sequential organ failure assessment (SOFA) and acute physiology and chronic health evaluation (APACHE) II score on admission], as well as clinical outcomes [such as, the duration of mechanical ventilation, ICU length of stay (LOS), length of hospital stay (LOHS), number of operative procedures, time to discharge alive, in-hospital mortality and 6-month-mortality]. As part of the daily data collection, the administration of BB and/or OX was documented as a dichotomous variable, since the specific type of BB and precise dosing of BB and OX were not documented in the RE-ENERGIZE study. The geographic region and characteristics of participating burn centers were also recorded.
All 1200 patients were included in this analysis and were stratified into four groups, based on the following inclusion criteria: BB (received at least one systemic administration of BB during hospitalization, without OX administration), OX (received systemic OX at least once during hospitalization, without BB administration), BBOX (received BB and OX each at least once during hospitalization), None (received neither systemic BB nor OX during hospitalization). No patients were excluded from this analysis.
Descriptive statistics and tests of significance were performed using GraphPad Prism Version 9.4.1. (La Jolla, CA). Continuous variables were tested for normality using the Kolmogorov–Smirnov test and reported as median and interquartile range if not normally distributed. Chi-squared testing was performed for categorical variables and Mann–Whitney tests were performed for continuous variables if not normally distributed.
We used a linear mixed effects model estimated by maximum likelihood with a fixed effect for region and a random effect for ICU, to test for significant variation in BB, OX and BBOX usage between regions and between ICUs within regions. Univariate regression analyses were used to determine predisposing demographic and clinical factors for the use of OX, BB or BBOX.
The primary endpoint of this analysis was time to discharge alive; secondary endpoints were acute and 6-month-mortality.
To investigate whether or not BB or OX were significant predictors of time to discharge alive, we used a Cox proportional hazards model with random frailty for ICU and we treated any death prior to 72 h after hospital discharge as a competing risk precluding discharge alive. Covariates included for adjustment were selected based on a priori considerations. We also used a Cox proportional hazards model with random frailty for ICU to model 6-month mortality after controlling for the same covariates. In both models we tested for an interaction (effect modification) between BB and OX and removed the interaction term if it was not statistically significant. Due to the exploratory nature of this analysis, we considered a two-sided p-value <0.05 without adjustment or multiplicity as indicative of statistical significance. All modelling was performed using SAS version 9.4 (SAS Institute Inc, Cary, NC, USA).
Results
All 1200 patients included in the primary analysis were included in the current analysis. Per geographic region of the respective burn center, the majority of patients in this trial were treated in the USA (56%), followed by Canada (17%), the EU (12%), UK (9%), Latin America (4%) and Asia (2%).
Demographics and baseline clinical variables by group stratification based on exposure to BB, OX, BBOX or None are summarized in Table 1. Of the included patients, 293 (24%) received BB alone, 282 (24%) received BBOX, 138 (12%) received OX alone and 487 (41%) received None.
Table 1.
Patient demographics
| All patients | OX | BB | BBOX | None | P-value | |
|---|---|---|---|---|---|---|
| n | 1200 | 138 | 293 | 282 | 487 | |
| Female (%) | 26 | 20 | 27 | 22 | 29 | 0.09 |
| Male (%) | 74 | 80 | 73 | 78 | 71 | 0.09 |
| TBSA (%) | 28 (20–40) | 37 (26–49) | 25 (20–40) | 35 (27–49) | 24 (19–33) | <0.0001 |
| Age (years) | 50 (34–63) | 48 (34–61) | 50 (33–65) | 44 (29–58) | 54 (37–65) | 0.0002 |
| BMI (kg/m2) | 27.1 (23.9–31.5) | 27.2 (24.0–30.8) | 26.7 (23.4–31.1) | 28 0.09 (24.7–33.6) | 26.6 (23.8–30.8) | 0.005 |
| CCI | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | 0 (0–1) | ≈ 0.99 |
| SOFA Score | 3 (1–5) | 2 (1–4) | 3 (1–5) | 3 (1–6) | 2 (0–5) | 0.006 |
| Apache II Score | 12 (8–20) | 14 (9–19) | 12 (7–19) | 17 (11–23) | 10 (7–17) | <0.0001 |
| Cause of injury | ||||||
| Scald (%) | 7.2 | 6.5 | 8.5 | 2.8 | 9.0 | 0.02 |
| Fire (%) | 89.9 | 90.6 | 88.4 | 95.7 | 87.3 | 0.004 |
| Chemical (%) | 2.4 | 2.2 | 2.4 | 1.4 | 3.1 | 0.71 |
| Other (%) | 0.5 | 0.7 | 0.7 | 0.00 | 0.6 | 0.76 |
| Ethnicity | ||||||
| White (%) | 75.7 | 73.2 | 78.2 | 76.6 | 74.3 | 0.73 |
| Black (%) | 8.1 | 13.0 | 10.2 | 8.5 | 5.13 | 0.02 |
| Hispanic (%) | 7.9 | 6.5 | 2.7 | 9.6 | 10.5 | 0.002 |
| Asian/Pacific Islander (%) | 4.3 | 2.9 | 4.1 | 2.1 | 6.2 | 0.09 |
| Native (%) | 2.6 | 1.5 | 3.1 | 2.5 | 2.7 | 0.91 |
| East Indian (%) | 0.3 | 0.7 | 0.3 | 0.0 | 0.4 | 0.80 |
| Other (%) | 1.1 | 2.2 | 1.4 | 0.7 | 0.8 | 0.66 |
All data are presented as median (interquartile range) or percentages (%). For more detailed descriptive demographics and baseline data see the original publication of the RE-ENERGIZE trial [24]. TBSA total body surface area, BMI body mass index, OX oxandrolone only, BB beta blocker only, CCI Charlson comorbidity index, APACHEacute physiology and chronic health evaluation score on admission, SOFA sequential organ failure assessment, BBOX concomitant BB and OX, None none of these drugs (BB, OX and BBOX)
Detailed drug administration data for BB, OX and BBOX are summarized in Table 2. BB and OX were started early after burn injury [BB: 5 (2–11) median (Q1–Q3) days post-burn (dpb); OX: 3 (2–8) dpb]. Relative to each patient’s individual LOHS, OX was started on average after 10% completion of LOS and ended after 92% completed LOS; BB was started at 17% LOHS and ended at 74% LOHS. The majority of patients receiving BB or OX continued to do so up to their last day of hospitalization (BB: 56%; OX: 65%).
Table 2.
Detailed administration data of OX, BB or BBOX
| All patients | OX | BB | BBOX | None | P-value | |
|---|---|---|---|---|---|---|
| Number of patients | 1200 | 138 | 293 | 282 | 487 | |
| LOHS, day | 31 (18–53) | 33 (20–58) | 33 (21–55) | 44 (27–68) | 23 (15–37) | <0.0001 |
| Earliest day on drug | 3 (2–8) | 5 (2–11) | 0.001 | |||
| Last day on drug | 26 (14–45) | 21 (13–34) | 0.02 | |||
| First day/LOHS | 10.2 (5.6–23.8) | 16.7 (7.8–34.6) | 0.002 | |||
| Last day/LOHS | 91.5 (71.5–100) | 74.5 (45.2–94.9) | <0.0001 | |||
| Days on drug (%) | 71.4 (46.1–88.9) | 38.5 (14.3–71.4) | <0.0001 | |||
| Drug until last day (%) | 65.2 | 54.9 | 0.047 |
Earliest day on drug and last day on drug are presented as absolute medians [interquartile range (IQR)]. First day/LOHS equals the first day of drug administration as a percentage of LOHS [median % (IQR)]. Last day/LOHS equals the day of drug administration as a percentage of LOHS [median % (IQR)]. Days on drug: percentage of days receiving OX, BB of LOHS [median (IQR)]. Drug until last day: percentage of patients receiving OX, BB on last day of hospitalization. LOHS length of hospital stay, OX oxandrolone only, BB beta blocker only, BBOX concomitant BB and OX, None none of these drugs (BB, OX and BBOX)
As shown in Table 1, factors associated with the administration of OX, BB or BBOX were being male, presenting with larger TBSA burns and a younger age than patients who received None (Table 1). Figure 1 shows the relative likelihood of receiving BB, OX, BBOX or None per geographic region and the respective absolute number of patients. Figure S1 (see online supplementary material) provides a world-map-visualization of BB, OX, BBOX or None use per geographic region. Table S1 (see online supplementary material) outlines high variability in administration of OX and BB not only per geographical region but also within various sites within each region. For example, the percentage of patients from sites in the USA that received BB ranged from 34.7 to 100% whereas the percentage that received OX ranged from 0 to 100%. Both the between region and between ICU variance were statistically significant at p < 0.01. Unadjusted analyses of predisposing factors for the use of OX, BB and BBOX are summarized in Table S2 (see online supplementary material). Overall, larger TBSA burns and worse APACHE II score on admission predisposed for use of BB, OX or BBOX over no medication. Male sex predisposed for BBOX and OX alone. Younger age and higher SOFA scores on admission predisposed for combination treatment with BBOX.
Figure 1.
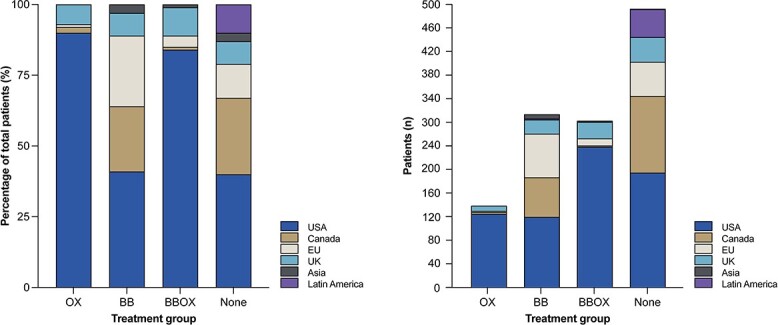
Geographical distribution of OX, BB, BBOX or None therapy use. Left: relative likelihood; right: absolute number of patients per region. OX oxandrolone only, BB beta blocker only, BBOX concomitant BB and OX, None none of these drugs (BB, OX and BBOX)
Association between OX and BB use and outcomes
When controlling for relevant covariates, the respective independent association of OX and BB administration, expressed as the proportion of hospital days receiving either OX or BB, and time to discharge alive, was modelled and is summarized in Table 3. There was no suggestion of BB or OX modifying each other’s association with time to discharge alive (test for interaction p-value = 0.57) so no interaction term was included in the model. A higher proportion of hospital days on OX (with or without BB) was associated with significantly slower time to discharge alive [hazard ratio (HR): 0.62 confidence interval (CI) (0.47–0.82), p = 0.001] per 100% increase, but BB (with or without OX) was not significantly associated with time to discharge alive. Time to discharge alive differed by geographic region. Being female and having higher TBSA, age, Charlson comorbidity index (CCI), Apache II score and SOFA score were all associated with slower time to discharge alive.
Table 3.
Multivariable Cox regression of time to discharge alive
| HR a | 95% CL | P -value | ||
|---|---|---|---|---|
| Geographical region | 0.0011 | |||
| Asia vs USA | 0.43 | 0.22 | 0.87 | |
| Canada vs USA | 0.80 | 0.53 | 1.20 | |
| EU vs USA | 0.79 | 0.54 | 1.16 | |
| Latin America vs USA | 0.34 | 0.16 | 0.70 | |
| UK vs USA | 0.80 | 0.52 | 1.26 | |
| Female vs. male | 0.75 | 0.65 | 0.88 | 0.0004 |
| TBSA | 0.95 | 0.95 | 0.96 | <.0001 |
| Age | 0.98 | 0.97 | 0.98 | <.0001 |
| CCI | 0.85 | 0.78 | 0.92 | <.0001 |
| Apache II score | 0.97 | 0.96 | 0.98 | <.0001 |
| BMI | 1.00 | 0.99 | 1.02 | 0.4385 |
| SOFA score | 0.90 | 0.88 | 0.93 | <.0001 |
| % Days on BBb | 0.98 | 0.77 | 1.25 | 0.8749 |
| % Days on OX | 0.62 | 0.47 | 0.82 | 0.0008 |
HR hazard ratio, CL confidence limits, TBSA total body surface area, BMI body mass index, OX oxandrolone only, BB beta blocker only, CCI Charlson comorbidity index, APACHE Aaute physiology and chronic health evaluation score on admission, SOFA sequential organ failure assessment, BBOX concomitant BB and OX, None none of these drugs (BB, OX and BBOX)
aHazard ratios <1 indicate slower time to discharge alive which can be due to higher mortality and/or longer hospital stay time
bThe interaction between days on BB and days on OX was tested and yielded p = 0.57, so it was excluded for model simplicity
Tables 4 and 5 repeat the prior analysis but with in-hopspital mortality and 6-month mortality as the outcome. Again, there is no suggestion of interaction between OX and BB (p = 0.87 and p = 0.62, resepectively) so the interaction term was not included in the model. After controlling for covariates, the in-hospital mortality HR for 0 vs. 100% days on BB was 0.5 (0.28–0.87, p = 0.015), There was no significant adjusted association between OX and in-hospital mortality. The 6-month mortality HR for 0 vs. 100% days on BB was 0.44 (0.24–0.82, p = 0.01). There was no significant adjusted association between OX and 6-month mortality. As with time to discharge alive, both in-hospital and 6-month mortality differed by region, and increasing TBSA, age, CCI and SOFA score were significantly associated with worse outcome. Figure 2 shows the unadjusted Kaplan–Meyer survival plots for all patients of all four groups.
Table 4.
Multivariable Cox regression of in-hospital mortality
| HR a | 95% CL | P -value | ||
|---|---|---|---|---|
| Geographical region | 0.0011 | |||
| Asia vs USA | 2.352 | 0.744 | 7.435 | |
| Canada vs USA | 0.549 | 0.321 | 0.940 | |
| EU vs USA | 0.913 | 0.522 | 1.597 | |
| Latin America vs USA | 5.589 | 4.009 | 7.791 | |
| UK vs USA | 0.707 | 0.304 | 1.641 | |
| Female vs male | 1.156 | 0.880 | 1.519 | 0.2965 |
| TBSA | 1.041 | 1.033 | 1.050 | <.0001 |
| Age | 1.061 | 1.045 | 1.077 | <.0001 |
| CCI | 1.136 | 1.038 | 1.243 | 0.0057 |
| Apache II score | 1.024 | 1.003 | 1.046 | 0.0225 |
| BMI | 1.013 | 0.981 | 1.047 | 0.4295 |
| SOFA score | 1.131 | 1.084 | 1.179 | <.0001 |
| % Days on BBb | 0.495 | 0.281 | 0.873 | 0.0152 |
| % Days on OX | 1.051 | 0.581 | 1.899 | 0.8699 |
HR hazard ratio, CL confidence limits, TBSA total body surface area, BMI body mass index, OX oxandrolone only, BB beta blocker only, CCI Charlson comorbidity index, APACHE acute physiology and chronic health evaluation score on admission, SOFA sequential organ failure assessment
aHR > 1 indicates increased hazard of mortality
bThe interaction between days on BB and days on OX was tested and yielded p = 0.87, so was excluded for model simplicity
Table 5.
Multivariable Cox regression of 6-month mortality
| HR a | 95% CL | P -value | ||
|---|---|---|---|---|
| Geographical region | <.0001 | |||
| Asia vs USA | 2.43 | 0.92 | 6.42 | |
| Canada vs USA | 0.58 | 0.30 | 1.11 | |
| EU vs USA | 0.90 | 0.48 | 1.69 | |
| Latin America vs USA | 5.63 | 2.47 | 12.82 | |
| UK vs USA | 0.73 | 0.37 | 1.44 | |
| Female vs male | 1.09 | 0.79 | 1.51 | 0.5825 |
| TBSA | 1.04 | 1.03 | 1.05 | <.0001 |
| Age | 1.06 | 1.05 | 1.07 | <.0001 |
| CCI | 1.14 | 1.05 | 1.23 | 0.0024 |
| Apache II score | 1.03 | 1.01 | 1.05 | 0.004 |
| BMI | 1.01 | 0.99 | 1.04 | 0.3185 |
| SOFA Score | 1.13 | 1.07 | 1.19 | <.0001 |
| % Days on BBb | 0.44 | 0.24 | 0.82 | 0.0099 |
| % Days on OX | 1.06 | 0.59 | 1.90 | 0.8448 |
HR hazard ratio, CL confidence limits, TBSA total body surface area, BMI body mass index, OX oxandrolone only, BB beta blocker only, CCI Charlson comorbidity index, APACHE acute physiology and chronic health evaluation score on admission, SOFA sequential organ failure assessment
aHR > 1 indicates increased hazard of mortality
bThe interaction between days on BB and days on OX was tested and yielded p = 0.62, so was excluded for model simplicity
Figure 2.
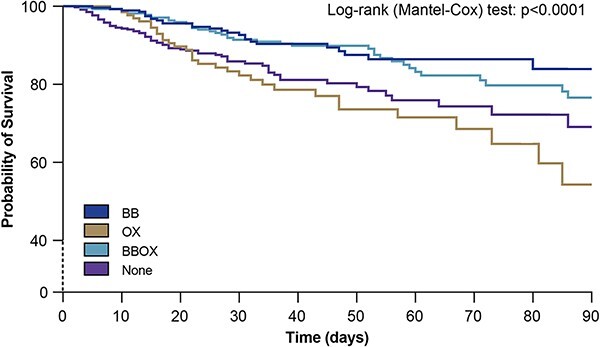
Unadjusted Kaplan–Meyer survival plot stratified per exposition to OX, BB, BBOX or None. OX oxandrolone only, BB beta blocker only, BBOX concomitant BB and OX, None none of these drugs (BB, OX and BBOX)
Discussion
The present study is the first to our knowledge to provide large-scale robust data about the clinical significance and usage patterns of BB, OX and combinations of the two drugs within a large collective of severely burned adults and among international burn centers. As 59% of patients in our international sample received either BB, OX or BBOX, we are able to confirm smaller studies that indicate that the use of BB, OX or BBOX is common in the treatment of severely burned adults, despite a persistent lack of conclusive data confirming their efficacy or safety in this patient population [25]. A recent survey of practice in the USA and a literature review indicate that a variety of adult burn centers have adopted regular usage of BB and OX administration [26, 27]. Our findings suggest that a large portion of burn care providers around the world are convinced that potential mitigation of hypermetabolism and hypercatabolism through BB and OX can improve patient outcomes enough to warrant administration. In this context, our unadjusted analyses revealed that there is a clear tendency to use these drugs in patients that are younger and more severely burned. This could be rooted in data from prospective RCTs that demonstrated favorable outcomes of BB and BBOX administration in severely or even massively burned children [8, 20]. It might also reflect the well-established notion that the degree of hypermetabolism and hypercatabolism correlates positively with TBSA [3]. The majority of patients in this study were treated in the USA, which is where OX was primarily used alone and in combination with BB, while prevalence of OX use in the EU and Asia was lower. One reason could be the availability of OX, which is significantly limited outside the USA and New Zealand and, outside of these countries, frequently necessitates more complicated and costly import via international pharmacies [13]. Accordingly, the use of more readily available BB was more evenly distributed throughout geographical regions worldwide.
Our data further show that administration of BB and OX was initiated early (5 and 3 dpb, respectively) post-burn, which is in line with the largest pediatric clinical trial to date, in which OX and BB were started on day 4 post-burn, after initial cardiocirculatory stabilization [21]. The duration of daily drug administration throughout a patient’s hospital stay varied between drug groups, with the highest adherence to OX treatment for almost three-quarters of patients’ hospitalization days. Of note, the majority of patients on either OX or BB received their respective drug on their last day before discharge from the hospital. The finding of relatively strong adherence to OX administration ties in with literature advocating for continuous and long-term use of anabolic agents to combat catabolism, which has been shown to persist for years in pediatric burn patients [14, 21, 28]. Extrapolation from these results and from other large studies in which sarcopenia is attenuated through oral OX administration (i.e cancer-related weight loss) might justify the administration patterns seen in the patient groups of this trial [29, 30]. Our unadjusted analyses of predisposing factors for the use of OX, BB or BBOX over none of the three indicate a tendency to use these drugs in younger and more severely injured patients, as TBSA and APACHE II scores and patient age at admission were significant predictors for OX, BB and BBOX use. The well-established positive pathophysiological correlation between burn severity (expressed via TBSA) and the extent of hypermetabolism, hyperinflammation and catabolism, respectively, might have driven individual treatment decisions for the use of either drug as observed in this study [6, 28, 31]. Nonetheless, one striking finding of this analysis is a high degree of variability regarding the OX and BB protocols, not only across geographical regions worldwide, but also across study sites within individual regions, such as the USA. This highlights the need for prospective studies to clearly determine potential therapeutic benefits and optimal dosing of BB, OX and BBOX. Otherwise, when considering the status quo, some patients may be harmed by ongoing over usage of these drugs or others harmed by underutilization of these therapeutic agents, and we will not know which until further trials are conducted.
For the first time in any large adult collective of severely burned adults, we present significant evidence that BB use might decrease mortality after severe burn injury. Our multivariable model adjusts for the most relevant confounders and uses the proportion of hospital days on BB as a robust and independent predictor of 6-month mortality. Naturally, these results need to be interpreted with caution, as singular interventions have become increasingly improbable to alter mortality on a large scale [32]. However, the fact that the observed effect size is rather large and remains significant in a large and heterogenous patient collective strongly encourages further investigation.
Several studies have shown improved acute and long-term physical recovery from severe burn injury following the use of OX to combat post-burn muscle wasting and aid in improved protein homeostasis [16, 20, 33]. In contrast with the largest RCT of OX use in severely burned adults with 81 participants [18] and numerous pediatric RCTs [33], we found that use of OX was associated with longer time to discharge alive. One possible explanation for this observation could be heterogeneous discharge regimens among burn centers around the world, i.e. with differing access to rehabilitation facilities, which in turn might necessitate longer inpatient care. In line with the aforementioned studies, OX use was not significantly associated with in-hospital or 6-month mortality. Wound healing times, which have been consistently reported to be shorter in burned children and adults receiving OX, were not available for analysis in this study.
This study has several limitations that bear consideration. Several data points were not available for analysis, as they were not collected in the RE-ENERGIZE trial, namely the exact type of BB administered, as well as exact doses of OX, BB or BBOX. Likewise, several endpoints that are essential to assess effects of BB, OX and BBOX on hypermetabolism, catabolism and hyperinflammation, such as LBM, muscle strength, REE, cardiac work and others, were not available from the RE-ENERGIZE data set. While there is very little probability of OX having been administered for a pre-existing medical condition and not in relation to the sustained acute burn injury, there might be patients in our data set who received BB for other reasons beyond specifically for their burn injury. This factor might bias inferences regarding individual treatment decisions. Likewise, predictors of treatment might lack some robustness, as some individual site characteristics regarding treatment decisions for OX, BB, BBOX or None were not available for analysis. Although we adjusted for several important covariates, the main limitation of this study, as with most observational studies, is that we cannot be sure if observed associations are causal due to the potential of residual confounding or potentially reverse causality. For example, it is conceivable that people who were expected to die early or to not stay long in the ICU were not prescribed BB or OX. Lastly, the fact that no significant differences in any primary, secondary and tertiary outcomes were detectable in the RE-ENERGIZE trial based on patient exposure to glutamine or placebo (main and supplementary results of [24]), made us confident to exclude exposition status to glutamine or placebo as a relevant confounder of the results presented in this trial. That notwithstanding, some physiological phenomena such as systemic inflammation were not assessed in the original data set and might thus have introduced some mixed and interactive bias to this post hoc analysis.
Despite these limitations we believe that the positive signals for BB and somewhat inconclusive results for OX were both derived from a sufficiently large patient collective, and are statistically robust and supported by a growing body of literature to serve as a stepping stone for further and more focused prospective randomized controlled trials into these pharmaceutical interventions.
Conclusions
By analyzing the largest available patient cohort of severely burned adults to date, we shed light on the current clinical use and significance of OX, BB and BBOX in international burn centers. We document considerable practice variability within and between regions of the world and delineate younger age and higher injury severity as predisposing factors for use of OX, BB and BBOX. We found that use of BB was significantly associated with reduced in-hospital- and 6-moth mortality but not time to discharge alive. Conversely, we found that use of OX was associated with longer ICU stay but not mortality. While our data remain inconclusive due to some methodological limitations, they are a strong argument for further research into these drugs, particularly given the high variability in treatment regimes across study sites worldwide. Ultimately BB and OX may be able to modulate the pathophysiological response to burn injury in severely burned adults, with significant effects on patient outcomes.
Abbreviations
APACHE II: Acute physiology and chronic health evaluation; BB: Beta-blocker; BBOX: Combination of beta blocker and oxandrolone; BMI: Body mass index; CCI: Charlson comorbidity index; CI: Confidence interval; dpb: Days post-burn; HLOS: Hospital length of stay; HR: Hazard ratio; ICU: Intensive care unit; IQR: Interquartile range; LBM: Lean body mass; Ox: Oxandrolone; RCT; Randomized controlled trial; REE: Resting energy expenditure; RE-ENERGIZE: RandomizEd Trial of ENtERal Glutamine to minimIZE Thermal Injury; SOFA: Sequential organ failure assessment; TBSA: Total body surface area.
Funding
Supported by the U.S. Department of Defense (award number, W81XWH-09-2-0194 for the pilot phase) and the Canadian Institutes of Health Research (funding reference numbers, MCT-94834 for the pilot phase and 14 238 for the definitive phase).
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors’ contributions
GH, EB, VM, CS and DKH were responsible for the study concept and design. EB, VM, AGD, AP, CT, VH, AP and AA were responsible for collecting the data. GH, AGD, AP, UK, BB and FD were responsible for statistical analysis. GH, DKH, AP, UK, CT, CS and FD were responsible for drafting the manuscript. GH, EB, VM, AGD, AP, CT, VH, UK, BB, ACP, AA, FD, CS and DKH were responsible for critical reading of the final version of the manuscript. All authors critically reviewed the manuscript and have approved the publication of this final version of the manuscript.
Ethics approval and consent to participate
This is a post hoc analysis of the RE-ENERGIZE (RandomizEd Trial of ENtERal Glutamine to minimIZE Thermal Injury, NCT00985205) trial [24]. The trial protocol was approved by the research ethics committees at Queen’s University and all participating centers. Informed consent/assent form was reviewed and approved by the Institutional Review Board (IRB).
Conflict of interest
None declared.
Supplementary Material
Contributor Information
Gabriel Hundeshagen, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Elisabeth Blears, Department of Plastic Surgery, Bayview Medical Center, Johns Hopkins University, 4940 Eastern Ave, Baltimore 21224, MD, USA.
Viktoria Mertin, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Andrew G Day, Kingston General Health Research Institute, Kingston Health Sciences Centre, 76 Stuart Street, Kingston, Ontario, K7L 2V7, Canada.
Alen Palackic, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Christian Tapking, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Valentin Haug, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Ulrich Kneser, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Björn Bliesener, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Adriana C Panayi, Department of Hand, Plastic and Reconstructive Surgery, Burn Center, BG Trauma Center Ludwigshafen, University of Heidelberg, Ludwig-Guttmann-Str. 13, 67071 Ludwigshafen, Germany.
Ariel Aballay, Department of Plastic Surgery, Bayview Medical Center, Johns Hopkins University, 4940 Eastern Ave, Baltimore 21224, MD, USA.
Francois Depret, Department of Anaesthesiology, Intensive Care Medicine and Burn center, Saint-Louis Hospital, 1 avenue Claude-Vellefaux, 75010, Asistance Publique Hôpitaux de Paris, Paris Cité University, France.
Christian Stoppe, Department of Anaesthesiology and Intensive Care Medicine, University Medical Center Schleswig-Holstein, Campus Kiel, Schwanenweg 21, 24105 Kiel, Germany; University Hospital, Würzburg, Department of Anesthesiology, Intensive Care, Emergency and Pain Medicine, Oberdürrbacher Str. 6, 97080 Würzburg, Germany; Departments of Cardiac Anesthesiology & Intensive Care Medicine, Charité Berlin, Augustenburger Platz 1 | 13353, Berlin, Germany.
Daren K Heyland, Department of Critical Care Medicine, Queen’s University, 76 Stuart Street, Kingston, K7L 2V7 Ontario, Canada.
References
- 1. Forjuoh SN. Burns in low- and middle-income countries: a review of available literature on descriptive epidemiology, risk factors, treatment, and prevention. Burns. 2006;32:529–37. [DOI] [PubMed] [Google Scholar]
- 2. Monk DN, Plank LD, Franch-Arcas G, Finn PJ, Streat SJ, Hill GL. Sequential changes in the metabolic response in critically injured patients during the first 25 days after blunt trauma. Ann Surg. 1996;223:395–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jeschke MG, Mlcak RP, Finnerty CC, Norbury WB, Gauglitz GG, Kulp GA, et al. . Burn size determines the inflammatory and hypermetabolic response. Crit Care. 2007;11:R90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakkar RK, Penatzer J, Simon S, Steele L, Fabia R, Groner JI, et al. . Measures of adaptive immune function predict the risk of nosocomial infection in Pediatric burn patients. J Burn Care Res Off Publ Am Burn Assoc. 2022;43:1416–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tapking C, Popp D, Herndon DN, Branski LK, Hundeshagen G, Armenta AM, et al. . Cardiac dysfunction in severely burned patients: current understanding of etiology, pathophysiology, and treatment. Shock. 2020;53:669–78. [DOI] [PubMed] [Google Scholar]
- 6. Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. . The pathophysiologic response to severe burn injury. Ann Surg. 2008;248:387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hassoun-Kheir N, Henig O, Avni T, Leibovici L, Paul M. The effect of β-blockers for burn patients on clinical outcomes: systematic review and meta-analysis. J Intensive Care Med. 2021;36:945–53. [DOI] [PubMed] [Google Scholar]
- 8. Herndon DN, Hart DW, Wolf SE, Chinkes DL, Wolfe RR. Reversal of catabolism by beta-blockade after severe burns. N Engl J Med. 2001;345:1223–9. [DOI] [PubMed] [Google Scholar]
- 9. Herndon DN, Barrow RE, Rutan TC, Minifee P, Jahoor F, Wolfe RR. Effect of propranolol administration on hemodynamic and metabolic responses of burned pediatric patients. Ann Surg. 1988;208:484–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Herndon DN, Rodriguez NA, Diaz EC, Hegde S, Jennings K, Mlcak RP, et al. . Long-term propranolol use in severely burned Pediatric patients: a randomized controlled study. Ann Surg. 2012;256:402–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Williams FN, Herndon DN, Kulp GA, Jeschke MG. Propranolol decreases cardiac work in a dose-dependent manner in severely burned children. Surgery. 2011;149:231–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Mohammadi AA, Bakhshaeekia A, Alibeigi P, Hasheminasab MJ, Tolide-ei HR, Tavakkolian AR, et al. . Efficacy of propranolol in wound healing for hospitalized burn patients. J Burn Care Res. 2009;30:1013–7. [DOI] [PubMed] [Google Scholar]
- 13. Garg A, Garg S, She RW. Development of an extemporaneous oral liquid formulation of oxandrolone and its stability evaluation. Burns. 2011;37:1150–3. [DOI] [PubMed] [Google Scholar]
- 14. Reeves PT, Herndon DN, Tanksley JD, Jennings K, Klein GL, Mlcak RP, et al. . Five-year outcomes after long-term OXANDROLONE administration in severely burned children: a randomized clinical trial. Shock Augusta Ga. 2016;45:367–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Porro LJ, Herndon DN, Rodriguez NA, Jennings K, Klein GL, Mlcak RP, et al. . Five-year outcomes after Oxandrolone Administration in Severely Burned Children: a randomized clinical trial of safety and efficacy. J Am Coll Surg. 2012;214:489–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Przkora R, Jeschke MG, Barrow RE, Suman OE, Meyer WJ, Finnerty CC, et al. . Metabolic and hormonal changes of severely burned children receiving long-term Oxandrolone treatment. Ann Surg. 2005;242:384–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ring J, Heinelt M, Sharma S, Letourneau S, Jeschke MG. Oxandrolone in the treatment of burn injuries: a systematic review and meta-analysis. J Burn Care Res. 2020;41:190–9. [DOI] [PubMed] [Google Scholar]
- 18. Wolf SE, Edelman LS, Kemalyan N, Donison L, Cross J, Underwood M, et al. . Effects of oxandrolone on outcome measures in the severely burned: a multicenter prospective randomized double-blind trial. J Burn Care Res Off Publ Am Burn Assoc. 2006;27:131–9discussion 140-141. [DOI] [PubMed] [Google Scholar]
- 19. Demling RH, Orgill DP. The anticatabolic and wound healing effects of the testosterone analog oxandrolone after severe burn injury. J Crit Care. 2000;15:12–7. [DOI] [PubMed] [Google Scholar]
- 20. Chao T, Porter C, Herndon DN, Siopi A, Ideker H, Mlcak RP, et al. . Propranolol and Oxandrolone therapy accelerated muscle recovery in burned children. Med Sci Sports Exerc. 2018;50:427–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Herndon DN, Capek KD, Ross E, Jay JW, Prasai A, El Ayadi A, et al. . Reduced Postburn hypertrophic scarring and improved physical recovery with yearlong Administration of Oxandrolone and Propranolol. Ann Surg. 2018;268:431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Van Zanten AR, Sztark F, Kaisers UX, Zielmann S, Felbinger TW, Sablotzki AR, et al. . High-protein enteral nutrition enriched with immune-modulating nutrients vs standard high-protein enteral nutrition and nosocomial infections in the ICU: a randomized clinical trial. JAMA. 2014;312:514–24. [DOI] [PubMed] [Google Scholar]
- 23. Heyland DK, Wischmeyer P, Jeschke MG, Wibbenmeyer L, Turgeon AF, Stelfox HT, et al. . A RandomizEd trial of ENtERal glutamine to minimIZE thermal injury (the RE-ENERGIZE trial): a clinical trial protocol. Scars Burns Heal. 2017;3:205951311774524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Heyland DK, Wibbenmeyer L, Pollack JA, Friedman B, Turgeon AF, Eshraghi N, et al. . A randomized trial of enteral glutamine for treatment of burn injuries. N Engl J Med. 2022;387:1001–10. [DOI] [PubMed] [Google Scholar]
- 25. Flores O, Stockton K, Roberts JA, Muller MJ, Paratz JD. The efficacy and safety of adrenergic blockade after burn injury: a systematic review and meta-analysis. J Trauma Acute Care Surg. 2016;80:146–55. [DOI] [PubMed] [Google Scholar]
- 26. LeCompte MT, Rae L, Kahn SA. A survey of the use of propranolol in burn centers: who, what, when, why. Burns. 2017;43:121–6. [DOI] [PubMed] [Google Scholar]
- 27. Brown DA, Gibbons J, Honari S, Klein MB, Pham TN, Gibran NS. Propranolol dosing practices in adult burn patients: implications for safety and efficacy. J Burn Care Res. 2016;37:e218–26. [DOI] [PubMed] [Google Scholar]
- 28. Jeschke MG, Gauglitz GG, Kulp GA, Finnerty CC, Williams FN, Kraft R, et al. . Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lesser GJ, Case D, Ottery F, McQuellon R, Choksi JK, Sanders G, et al. . A phase III randomized study comparing the effects of oxandrolone (ox) and megestrol acetate (meg) on lean body mass (LBM), weight (wt) and quality of life (QOL) in patients with solid tumors and weight loss receiving chemotherapy. J Clin Oncol. 2008;26:9513–3. [Google Scholar]
- 30. Tchekmedyian S, Fesen M, Price LM, Ottery FD. Ongoing placebo-controlled study of oxandrolone in cancer-related weight loss. Int J Radiat Oncol Biol Phys. 2003;57:S283–4. [Google Scholar]
- 31. Herndon DN, Wilmore DW, MasonAD, Jr. Development and analysis of a small animal model simulating the human postburn hypermetabolic response. J Surg Res. 1978;25:394–403. [DOI] [PubMed] [Google Scholar]
- 32. Pereira C, Murphy K, Herndon D. Outcome measures in burn care: is mortality dead? Burns. 2004;30:761–71. [DOI] [PubMed] [Google Scholar]
- 33. Li H, Guo Y, Yang Z, Roy M, Guo Q. The efficacy and safety of oxandrolone treatment for patients with severe burns: a systematic review and meta-analysis. Burns J Int Soc Burn Inj. 2016;42:717–27. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.


