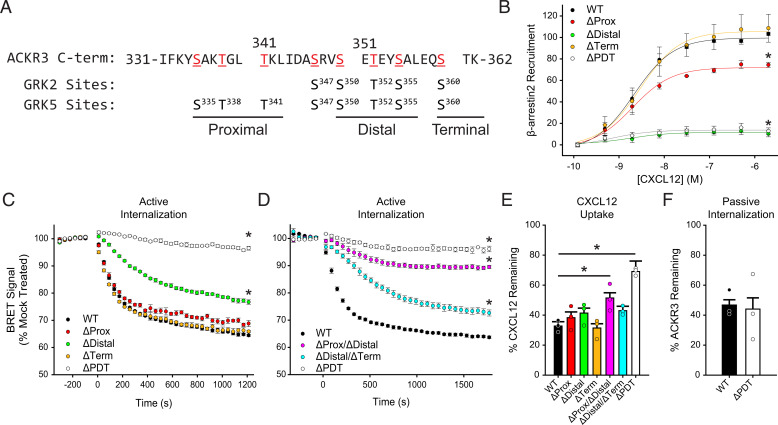
Fig. 4.
Specific phosphorylation motifs differently contribute to CXCL12 responses by ACKR3 in HEK293A cells. (A) ACKR3 was phosphorylated by either GRK2 or GRK5 in vitro, and the specific sites of modification were determined by mass spectrometry. The detected phosphorylation sites are highlighted in red. Phosphorylated positions in the ACKR3 C-terminus were divided into three clusters and replaced by alanine to produce ΔProx, ΔDistal, ΔTerm, and ΔPDT receptor constructs. (B) Recruitment of GFP_βarr2 to phosphorylation-deficient ACKR3 constructs expressed in WT HEK293A cells and tested across a titration of CXCL12 and detected by BRET. Values represent three independent experiments performed in triplicate and normalized to WT ACKR3 recruitment. (C and D) Active internalization of individual (C) and multiple (D) phosphorylation cluster substitutions tracked by the loss of BRET between ACKR3_RlucII and rGFP_CAAX after CXCL12 addition. (E) CXCL12 uptake by phosphorylation deficient ACKR3 constructs, measured by remaining chemokine and compared with cells transfected with empty vector. (F) Passive agonist-independent internalization of the triple phosphorylation substitution observed by the prelabeled flow cytometry experiment. WT ACKR3 data in B, C, E, and F were repeated from Figs. 1 and 2A for comparison. All error bars represent standard deviations, and statistical significance was determined by (B) the extra sum of squares F-test and (C, D, and E) one-way ANOVA followed by a Bonferroni test. *P < 0.001.