Figure 4. A structural basis for regulation of TIM nuclear entry.
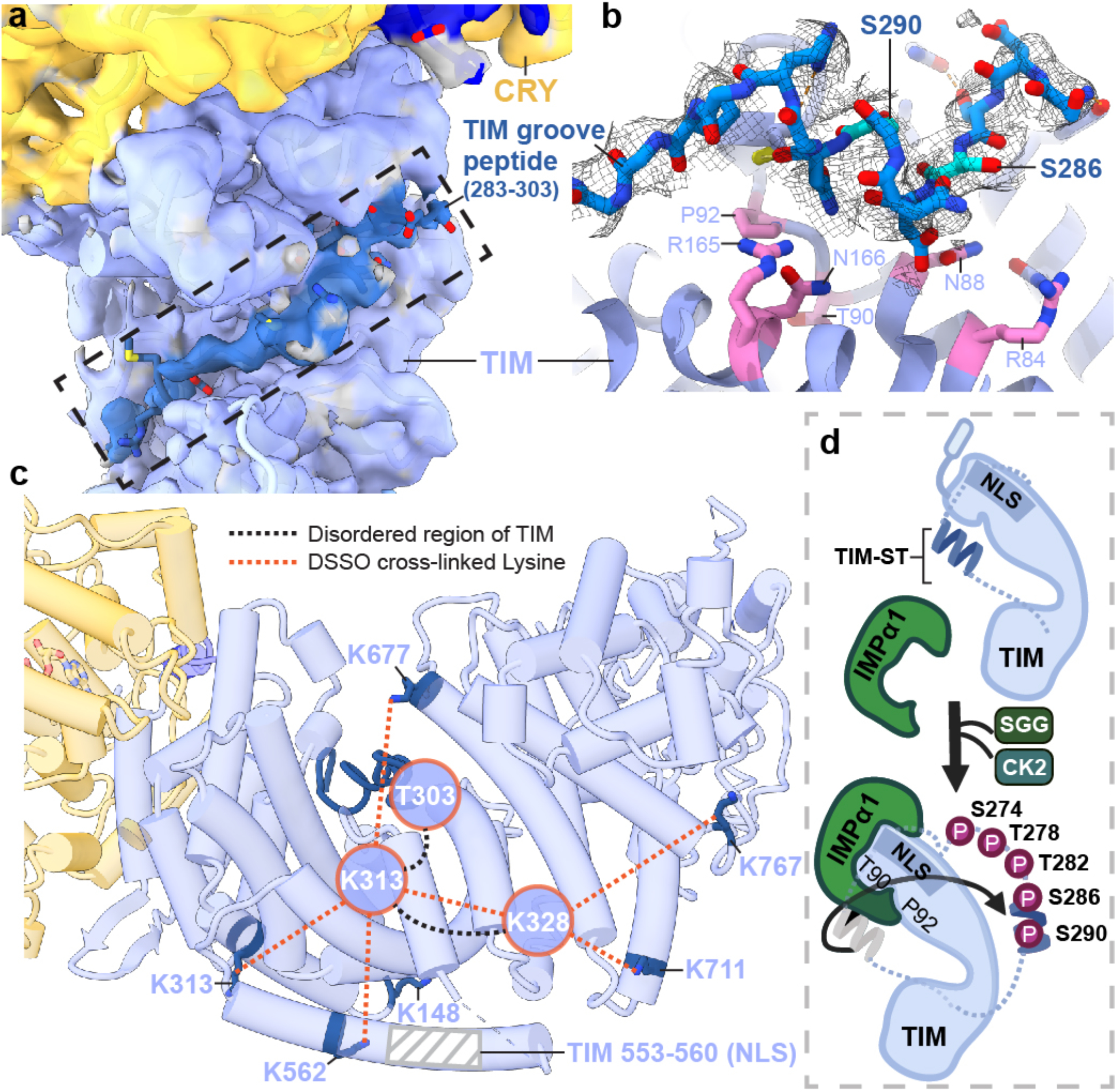
(a) Electron density (dark blue) for an extended polypeptide resides within a groove wherein members of the β-catenin and α-importin proteins bind their target sequences. (b) The TIM groove peptide density shown with the sequence assignment S283-T303. The peptide interacts with β-catenin-conserved Asn and Arg residues and buries residues important for importin-α1 binding (T90 and P92). (c) DSSO-mediated lysine crosslinking patterns of disordered residues K313 and K238 shown on the TIM structure. K313 and K328 produce a pattern of crosslinking that localizes them to a position consistent with placement of the S283-T303 groove peptide. The TIM NLS lies in a helix adjacent to the groove but does not occlude T90 and P92. (d) Schematic of proposed nuclear entry mechanism of TIM. Phosphorylation of the TIM-ST region by SGG and CK2 releases the ST region from TIM groove to expose TIM T90 and P92. Importin-α1 binds TIM adjacent to Thr90 and Pro92, while also binding the TIM NLS within a structurally analogous ARM groove of its own.
