Extended Data Figure 4. Key interactions at the CRY:TIM interface.
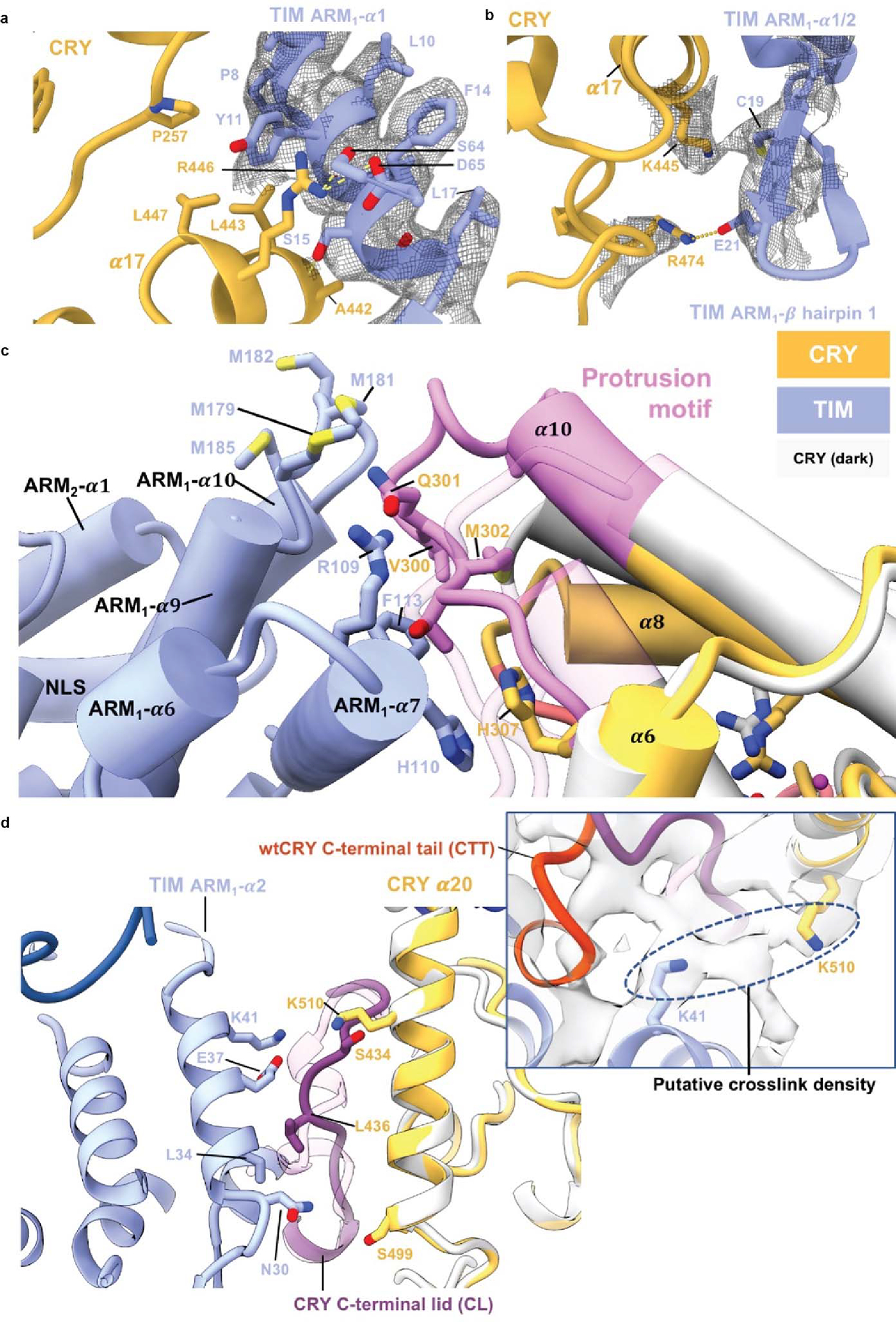
(a) Interactions between the bottom half of TIM ARM1-α1 with CRY. ARM1- α1 binds across CRY α14 and α17. The guanidinium of CRY R446 (on α17) forms π-cation interaction with TIM Y11. TIM Y11 participates in hydrophobic contacts with CRY P257, L443 and L447. On TIM α1, TIM P8 stacks against CRY α12 and TIM S15 hydrogen bonds to the backbone carbonyl of CRY A442. (b) In the β-hairpin connecting ARM1- α1 to ARM1- α2 E21 salt-bridges to R474 and C19 interacts with K445. (c) Interactions of the CRY protrusion motif. TIM R109, F113, and H110 interact with the protrusion motif of CRY. TIM ARM2-α3 H110 stacks with CRY H307. Four Met residues (M179, M181 M182, M185) in the ARM3-α2-α3 loop provide side-chain interactions with the tip of the CRY protrusion motif. The CRY α10 helix lengthens to residue A295 compared to the dark state and the following 300–306 residues interact directly with ARM2-α3. (d) Interactions of the CRY C-terminal lid and α20 helix. The restructured C-terminal lid interacts with residues on TIM ARM1- α2. For examples, L34 make a hydrophobic contact with I436 and E37 hydrogen bonds to the backbone of S434. At the end of ARM1- α2, Asn30 contacts the N-terminal end of CRY α20. A DSSO crosslink observed between Lys510 on CRY a20 and Lys41 on ARM1-α3 is fully consistent with the observed interface.
