Extended Data Figure 5. Changes in flavin binding pocket for CRY dark and bound states.
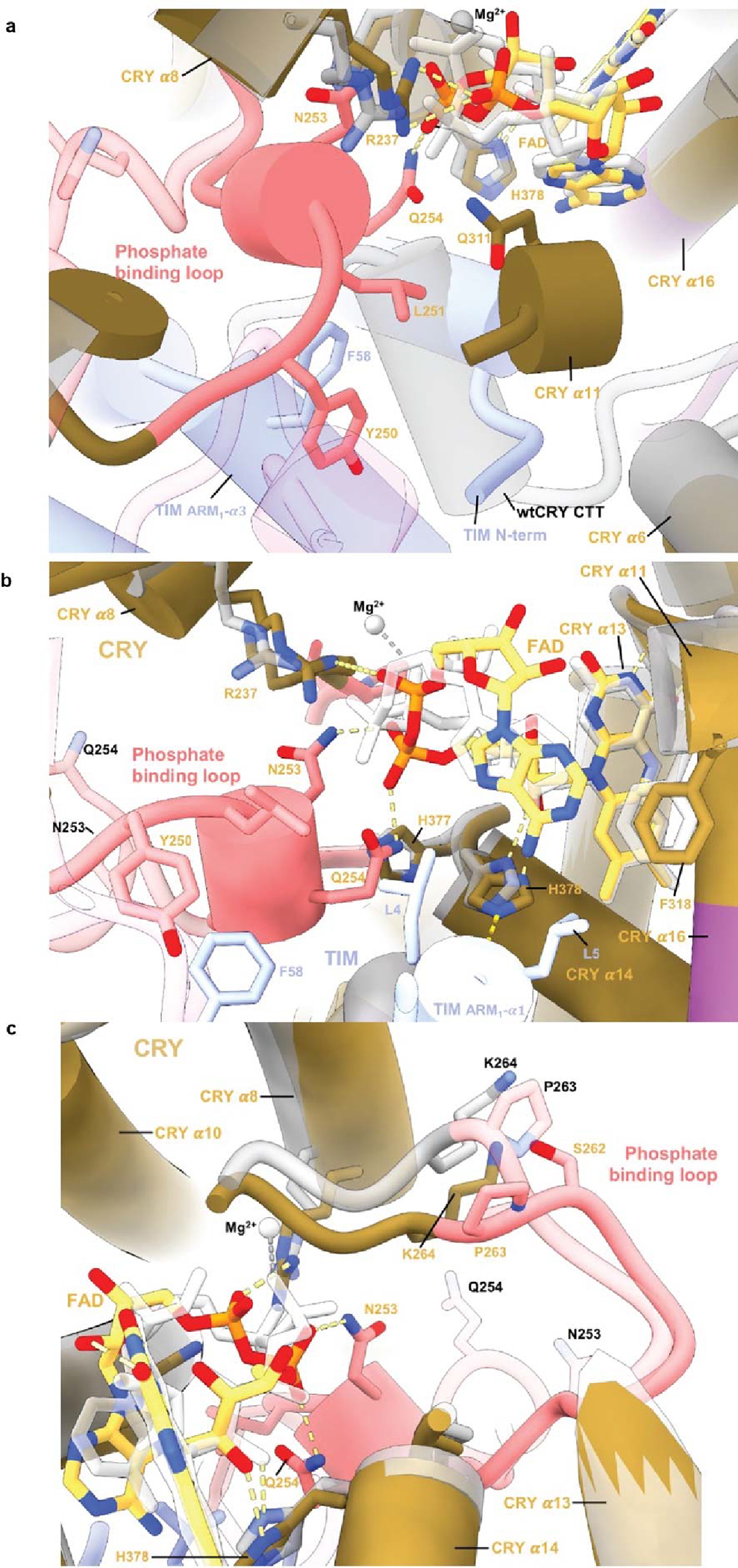
Comparison of the flavin pocket in CRY:TIM compared to in dark-state CRY (PDB 4GU5). (a) In CRY:TIM, Y250 and L251 interact with the FAD pocket and make space for the top of TIM ARM1-α3 to insert F58 behind the new conformation of the phosphate-binding loop. (b) The FAD adenine ring of CRY:TIM slides 1.5 Å relative to the unbound structure. CRY F318 shifts away from its original position and harbors helix α11 to compensate for the FAD adenine ring adjustment, following the protrusion motif. (c) G311 swivels away from the inserted TIM L4. New diphosphate conformations cause rearrangement of the CRY 262-to-264 loop.
