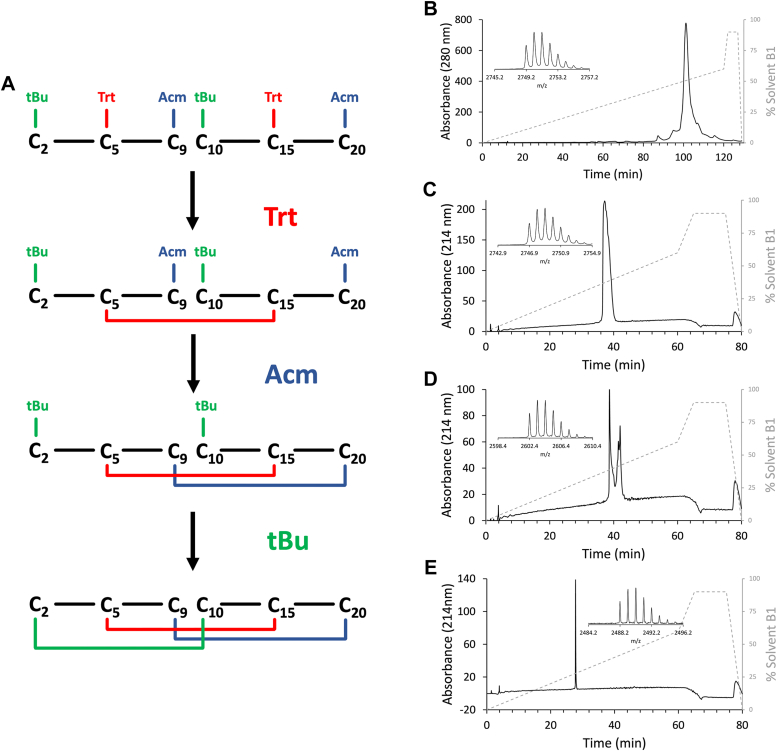
Figure 4.
TxVIIB stepwise oxidation strategy and RP-HPLC spectra of regioselective disulfide-bond formation.A, schematic procedure used for protected TxVIIB synthesis. B, preparative RP-HPLC of the reduced crude linear TxVIIB used for direct disulfide formation strategy. Inset: MS spectrum of the synthetic compound with the Acm and tBu protecting groups. [M + H]+ at m/z 2749.2 (theoretical [M + H]+ = 2749.3, error 36.4 ppm). C, analytical RP-HPLC of purified TxVIIB with the first disulfide bond, Cys5-Cys15. Inset: MS spectrum of the compound [M + H]+ at m/z 2746.9 (theoretical [M + H]+ = 2747.3, error 145.6 ppm). D, analytical RP-HPLC of purified TxVIIB with its first two disulfide bonds (oxidation of Cys9-Cys20). The two peaks represent the two isomers of this conformational stage. Inset: MS spectrum of the compound [M + H]+ at m/z 2602.4 (theoretical [M + H]+ = 2603.1, error 268.9 ppm). E, analytical RP-HPLC of purified TxVIIB with in its fully folded conformation (last disulfide bond Cys2-Cys10). Inset: MS spectrum of the compound [M + H]+ at m/z 2488.2 (theoretical [M + H]+ = 2488.9, error 281.2 ppm). MS, mass spectrometry; RP-HPLC, reversed-phase high performance liquid chromatography.