Table 3.
Comparison of the Counts and Positive Rate of E/M-CTCs in Different Clinical Feature Groups
| E/M-CTC Counts | Positive Rate of E/M-CTCs (%) | P-value | ||||
|---|---|---|---|---|---|---|
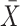 ± SD ± SD |
T-test | Median | Mann–Whitney | |||
| Age | 0.587 | 0.090 | ||||
| ≤ 63 | 9.13 ± 7.108 | 100 | ||||
| >63 | 10.86 ± 10.068 | 78.6 | ||||
| Sex | 0.587 | >0.999 | ||||
| Male | 9.13 ± 8.469 | 87.5 | ||||
| Female | 10.86±8.769 | 92.9 | ||||
| Smoking | 0.599 | >0.999 | ||||
| No | 0 (0~3) | 92.3 | ||||
| Yes | 0 (0~1) | 88.2 | ||||
| Pathological type | 0.783 | 0.716 | ||||
| ADC | 9.67 ± 9.484 | 81.8 | ||||
| SCC | 9.27 ± 6.930 | 93.3 | ||||
| Others | 12.75 ± 10.340 | 100 | ||||
| TNM stage | 0.0001 | 0.070 | ||||
| I-II | 3.0(0.0~8.0) | 76.9 | ||||
| III-IV | 13.0(6.0~30.0) | 100 | ||||
| Tumor size stage | 0.0125 | >0.999 | ||||
| T1-T2 | 7.0(0.0~30.0) | 87.5 | ||||
| T3-T4 | 18.5(6.0~30.0) | 100 | ||||
| Lymph node metastasis | 0.0010 | 70 | 0.030 | |||
| No | 2.0(0.0~15.0) | 100 | ||||
| Yes | 9.5(3.0~30.0) | |||||
| Distant metastasis | 0.0039 | 0.534 | ||||
| No | 6.0(0.0~30.0) | 85.7 | ||||
| Yes | 15.0(6.0~30.0) | 100 | ||||
Notes: The positive rate of E/M-CTCs is referred to the proportion of the CTC cells with positive epithelial marker and mesenchymal marker signals (epithelial marker+, mesenchymal marker+, CD45-, and DAPI+) by immunofluorescence among the total number of CTC cells.
Abbreviations: E/M-CTCs, hybrid epithelial/mesenchymal circulating tumor cells; ADC, adenocarcinoma; SCC, squamous cell carcinoma; TNM, tumor-node-metastasis.
