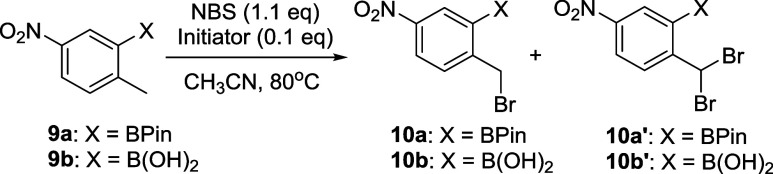
Table 3. Radical Bromination of 9a/b for the Synthesis of Bromide 10a/ba.
| reactant | radical initiator | monobromo product (LCAP) | dibromo product (LCAP) |
|---|---|---|---|
| 9a | AIBN | 10a: 90%b | 10a′: <5% |
| 9b | AIBN | 10b: 85%c | 10b′: 15% |
| 9a | BPO | 10a: 82%b | 10a′: <5% |
| 9b | BPO | 10b: 50%c | 10b′: 30% |
| 9b | incandescent light | 10b: 80%c | 10b′: 10% |
All reactions were performed with 9a or 9b (1 g, 1 equiv), NBS (1.1 equiv), initiator (0.1 equiv), or incandescent light at 80 °C in acetonitrile for 8 h.
LCAP of the crude residue without trituration.
LCAP of the product triturated from water.