Table 4. Synthesis of 6-Amino-1-hydroxy-2,1-benzoxaborolane 6 by Reduction of 12 under Continuous Flow Conditionsa.
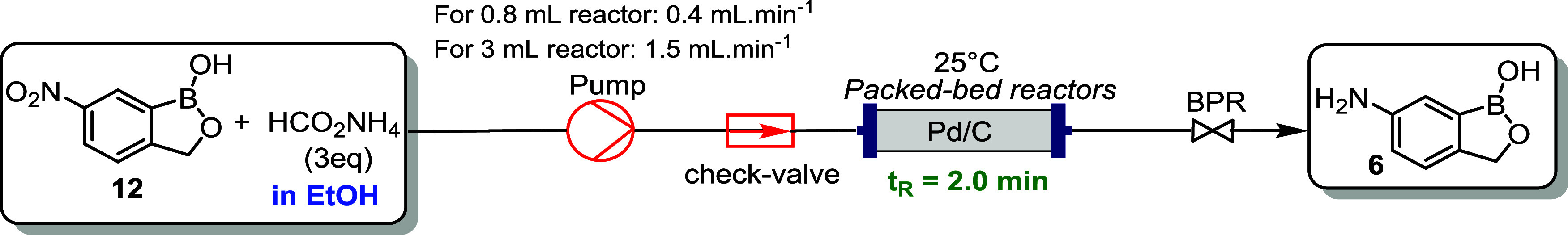
All reactions were performed with 12 (0.5 g, 2.8 mmol, 1.0 equiv), Pd/C, and HCO2NH4 (3 equiv) in EtOH (20 mL, 40 V), at 25 °C.
Isolated yield.
In a 1 mL reactor, flow rate: 0.1 mL/min.
In a 1 mL reactor, flow rate: 0.4 mL/min.
Assay yield based on 1H NMR, 55% of 12 remained.
In a 3 mL reactor, flow rate: 1.5 mL/min, 5 g of 12 was used, and 6 was obtained with >99% HPLC purity after a trituration.
