“It is impossible to determine with the naked eye whether or not the disease has extended into the pectoral muscle” - William Stewart Halsted, on breast cancer, 1890 [1]
Just as William Stewart Halsted, the Father of American Surgery, noted more than 125 years ago, surgeons continue to play a critical role in the care of patients with solid tumors. Today, 65% of patients with cancer still undergo surgery with a curative intent [2]. Yet, of the roughly 800,000 patients that undergo cancer surgery each year, up to 40% will recur [2]. Many of these patients recur within 2 centimeters of the surgical resection margins. When these patients do recur, the outcomes are dismal, and the vast majority of these individuals die within 2 years [2]. Despite surgeons knowing the importance of a thorough and complete operation, one must wonder why the failure rate of cancer operations remains so high. When considering the challenges that a surgical oncologist must overcome to ensure a successful operation, one better appreciates the high failure rate.
During a cancer operation, the surgeon must make three critical oncologic decisions:
How much to resect? After exposing the tumor, the surgeon must estimate the extent of its margins even when they cannot be visualized or palpated.
Which lymph nodes should be removed? During the operation, the surgeon must identify and remove lymph nodes which may harbor invasive cancer cells in order to clear disease and improve staging.
Is synchronous or metastatic disease present? The surgeon must search the exposed body cavity for suspicious lesions and determine if these areas should be resected.
Even 125 years ago, Halsted appreciated these challenges. He described, “the breast tumor was circumscribed by a skin incision which gave the diseased tissues at every point a wide berth – a berth of at least 5 cm”.[1] He chose 5 cm because he believed it to be an adequate margin. Halsted also preferred to remove all axillary nodes, because it was impossible to determine exactly which nodes might contain cancer: “…the axillary contents and with them all the cellular tissue and fat which covers the front and side of the exposed chest wall were dissected off” [1]. Halsted’s intuition that there must be satellite cancer cells in the underlying muscle led him to become an advocate for the radical mastectomy: “From the careful microscopic examination of many very small cancers of the breast I am convinced that the pectoralis major is usually, at the time of the operation, involved in new growth” and he chose to “excise in almost every case the pectoralis major muscle…”[1] Over a century later, the modern surgeon’s ability to correctly make these three critical decisions remains the most important factors in determining the patient’s outcome.
Atul Gawande claims that surgery has become ‘radically more effective’ over the past two hundred years [3]. However, to this day, over a century after Halsted’s era, surgical oncologists still rely almost exclusively on the same two tools to make intra-operative decisions: their hands for palpating tumors and their eyes to detect abnormal planes, tissues and contours. When we look at the results following cancer surgery, it becomes even more evident that the pace of cancer surgery progress has been discouraging.
How do we help the surgical oncologist make better choices? The answer lies in development of additional tools capable of real-time tumor localization and identification. A variety of methods have been utilized to increase the diagnostic yield of the surgical oncologist and include technologies such as percutaneous wire placement, lymph node mapping, intraoperative ultrasound and fluoroscopy and even MRI scans [4]. These approaches, however, are associated with several potential pitfalls (such as exposure to wire-displacement, ionizing radiation, hematoma, etc.) which limit widespread adoption. Furthermore, these methods are often expensive, time-consuming and require extensive training [4].
A rapidly evolving approach that may better address the surgical oncologist’s needs is “intraoperative molecular imaging” (IMI), or the utilization of systemically administered optical contrast agents which can enhance the surgeon’s ability to detect disease. There are several imaging agents which have been developed to accumulate in tumors by targeting tumor-specific molecular differences or changes within the tumor microenvironment. These agents are typically administered anywhere from several days to several hours prior to resection [5, 6]. During resection, fluorescent images are generated in real time (Figure 1); thus little additional time is required for intraoperative assessment. In fact, a recent report by our group has noted that lesions can be accurately identified and characterized in an average of 2.4 minutes, which is nearly 10 times quicker than with traditional frozen-section analysis [7].
Figure 1: Intraoperative Molecular Imaging has wide range applicability to the surgical oncologist:
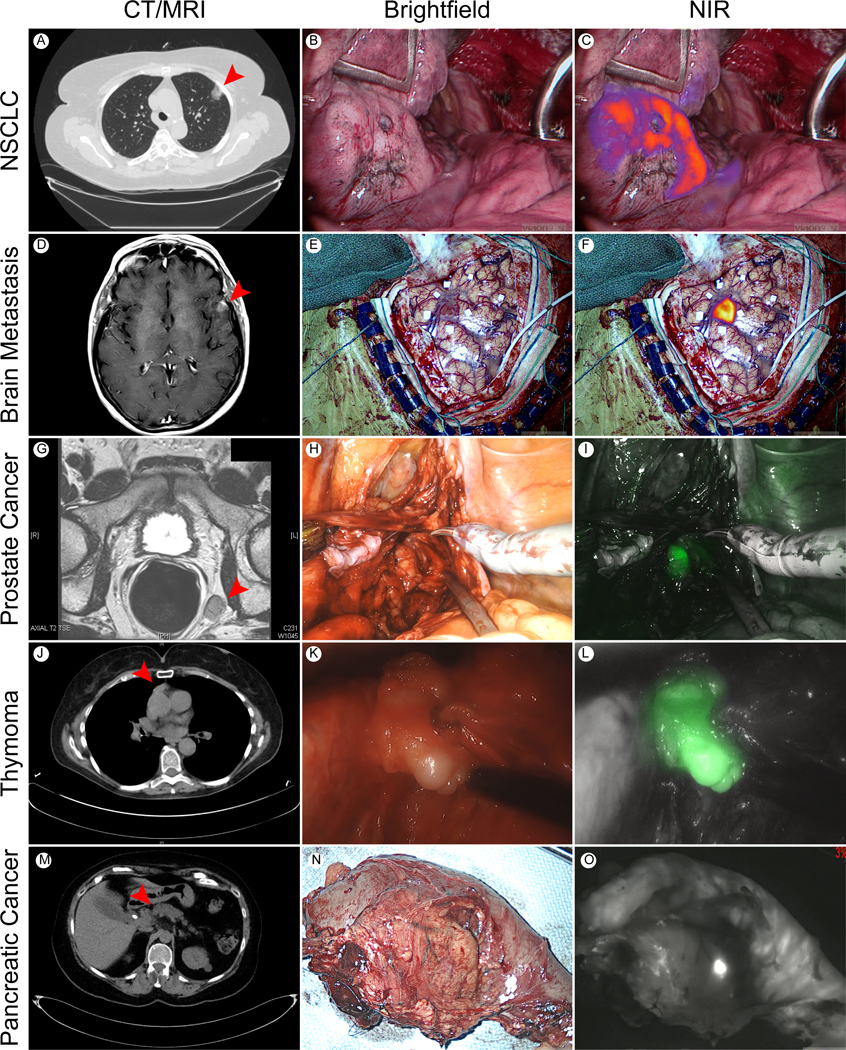
(A) CT Chest demonstrating Left Upper Lobe NSCLC. (B) Brightfield and (C) near infrared (NIR) images of Left Upper Lung NSCLC nodule in subject receiving the folate targeted tracer, OTL0038. (D) CT head revealing left hemispheric NSCLC metastasis. (E) Brightfield and (F) fluorescent image of subject receiving the non-specific NIR tracer, indocyanine green (ICG). (G) Pelvic MRI demonstrating concerning left iliac lymph node enlargement in patient with history of prostatic cancer. (H) Brightfield and (I) fluorescent images of iliac lymph node harboring metastatic prostate cancer located during robotic surgery. Subject received ICG prior to resection. (J) CT Chest visualizing mediastinal mass anterior to ascending aorta. (K) Brightfield and (L) NIR images of thymoma at time of transcervical resection. Subject received ICG prior to thymectomy. (M) CT abdomen localizing pancreatic adenocarcinoma in pancreatic head (N) Brightfield and (O) NIR image of Whipple specimen in subject receiving ICG prior to resection. Red arrowhead points to tumor in axial imaging.
The authors of this manuscript are members of the United States’ largest center for image guided surgery and have recently enrolled their 500th subject in an intraoperative imaging trial. Broadly, the group has preliminary success imaging thoracic, gastrointestinal, neurologic and genitourinary malignancies (Figure 1) [5, 8, 9]. In addition to these successes, Phase I/II trials are active for a wide range of common malignancies including brain, pituitary, ovarian, testicular, colorectal, gastric, esophageal, pancreatic, hepatocellular, renal, renal, bladder, prostate, parathyroid and thyroid [10, 11]. Furthermore, there have been encouraging results described for less common malignancies such as mesothelioma [12], thymoma [13] and sarcoma [14].
Due to heterogeneity in approaches and the early stages of this technology, it is challenging to generate conclusions about clinical impact. However, several important lessons have been learned: intraoperative imaging is associated with minimal toxicity, capable of detecting positive margins and distant metastases, accurate in detecting involved lymph nodes, and sensitive to the subcentimeter range [7, 15–17]. By examining results from two of the most systematically examined approaches, imaging of glioblastoma multiforme (GBM) with 5-aminoleuvolinic acid (5-ALA) and NSCLC with folate-targeted agents, one may begin to appreciate potential clinical utility. To elaborate, in Phase III evaluations involving 5-ALA for imaging of GBM, authors have noted increases in complete resection rates (65% versus 36%) and improved progression free survival at 6-months (41% versus 21%) [18]. Further, sensitivities and specificities with this approach were 77% and 92%, respectively; while positive predictive values and negative predictive values were 92% and 79%, respectively [19]. In the authors’ experiences involving folate-targeted tracers for pulmonary adenocarcinomas, a remarkable 100% accuracy has been noted [7]. Perhaps more impressively, folate-targeted imaging has helped surgeons detect nodules that were not appreciated by preoperative imaging or by traditional intraoperative localization techniques in nearly 15% of subjects [5]. These encouraging results have been echoed by dozens of other preliminary institutional experiences that suggest marked improvements in the surgeon’s decision making ability [20, 21].
In order for this technology to make further progress, it is critical to develop new strategies to overcome hurdles including variable dynamic range, poor depth of penetration and tumor heterogeneity. Most importantly, the impact of intraoperative imaging on patient outcomes will depend on an acceptance of a new technology, something that is often difficult for the surgeon. Until we integrate such technologic advances into our operating rooms, cancer surgeons will continue to face the same struggles which burdened Halsted. With real-time imaging, we can alleviate these century-old struggles and ultimately save lives.
Funding Sources:
JDP was supported by a grant by the American Philosophical Society, and an NIH F32 (1F32CA210409), and an Association for Academic Surgery Research Grant. SS was supported by an NIH R01 (R01 CA193556).
Footnotes
Disclosures: The authors have no disclosures to report. JYKL owns stock options in an imaging company – VisionSenseTM.
References:
- 1.Halsted WS, Surgical Papers. Second ed. 1952, Wakefield, MA: The Murray Printing Company. [Google Scholar]
- 2.Aliperti LA, et al. , Local and systemic recurrence is the Achilles heel of cancer surgery. Ann Surg Oncol, 2011. 18(3): p. 603–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gawande A, Two hundred years of surgery. N Engl J Med, 2012. 366(18): p. 1716–23. [DOI] [PubMed] [Google Scholar]
- 4.Keating J and Singhal S, Novel Methods of Intraoperative Localization and Margin Assessment of Pulmonary Nodules. Semin Thorac Cardiovasc Surg, 2016. 28(1): p. 127–36. [DOI] [PubMed] [Google Scholar]
- 5.Predina JDK,J; Gaughan C; Jarrar D; Pechet T; Kucharczuk J; Low P; Singhal S, A Phase I Clinical Trial of Targeted Intraoperative Molecular Imaging for Pulmonary Adenocarcinomas, in STS 53rd Annual Meeting. 2017: Houston, TX, USA. [Google Scholar]
- 6.Rosenthal EL, et al. , Safety and Tumor Specificity of Cetuximab-IRDye800 for Surgical Navigation in Head and Neck Cancer. Clin Cancer Res, 2015. 21(16): p. 3658–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kennedy GT, et al. , The Optical Biopsy: A Novel Technique for Rapid Intraoperative Diagnosis of Primary Pulmonary Adenocarcinomas. Ann Surg, 2015. 262(4): p. 602–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guzzo TJ, et al. , Intraoperative Molecular Diagnostic Imaging Can Identify Renal Cell Carcinoma. J Urol, 2016. 195(3): p. 748–55. [DOI] [PubMed] [Google Scholar]
- 9.Lee JY, et al. , Intraoperative Near-Infrared Optical Imaging Can Localize Gadolinium-Enhancing Gliomas During Surgery . Neurosurgery, 2016. 79(6): p. 856–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tipirneni KE, et al. , Oncologic Procedures Amenable to Fluorescence-guided Surgery. Ann Surg, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tipirneni KE, et al. , Fluorescence Imaging for Cancer Screening and Surveillance. Mol Imaging Biol, 2017. [DOI] [PubMed] [Google Scholar]
- 12.Predina J NA, Keating J, Dunbar A, Connolly C, Singhal S, Near-Infrared Intraoperative Molecular Imaging Can Enhance Malignant Pleural Mesothelioma Resection, in The 25th Annual Meeting of the Asian Society for Cardiovascular and Thoracic Surgery. 2017: Seoul, Korea. [Google Scholar]
- 13.Keating JJ, et al. , Intraoperative imaging identifies thymoma margins following neoadjuvant chemotherapy. Oncotarget, 2016. 7(3): p. 3059–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Keating J, et al. , Near-Infrared Intraoperative Molecular Imaging Can Locate Metastases to the Lung. Ann Thorac Surg, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keating J, et al. , Identification of breast cancer margins using intraoperative near-infrared imaging. J Surg Oncol, 2016. 113(5): p. 508–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia L, et al. , Intraoperative Molecular Imaging for Post-Chemotherapy Robot-Assisted Laparoscopic Resection of Seminoma Metastasis: A Case Report. Clin Genitourin Cancer, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Keating JJ, Kennedy GT, and Singhal S, Identification of a subcentimeter pulmonary adenocarcinoma using intraoperative near-infrared imaging during video-assisted thoracoscopic surgery. J Thorac Cardiovasc Surg, 2015. 149(3): p. e51–3. [DOI] [PubMed] [Google Scholar]
- 18.Stummer W, et al. , Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol, 2006. 7(5): p. 392–401. [DOI] [PubMed] [Google Scholar]
- 19.Colditz MJ and Jeffree RL, Aminolevulinic acid (ALA)-protoporphyrin IX fluorescence guided tumour resection. Part 1: Clinical, radiological and pathological studies. J Clin Neurosci, 2012. 19(11): p. 1471–4. [DOI] [PubMed] [Google Scholar]
- 20.van Dam GM, et al. , Intraoperative tumor-specific fluorescence imaging in ovarian cancer by folate receptor-alpha targeting: first in-human results. Nat Med, 2011. 17(10): p. 1315–9. [DOI] [PubMed] [Google Scholar]
- 21.van der Vorst JR, et al. , Near-infrared fluorescence-guided resection of colorectal liver metastases. Cancer, 2013. 119(18): p. 3411–8. [DOI] [PMC free article] [PubMed] [Google Scholar]


