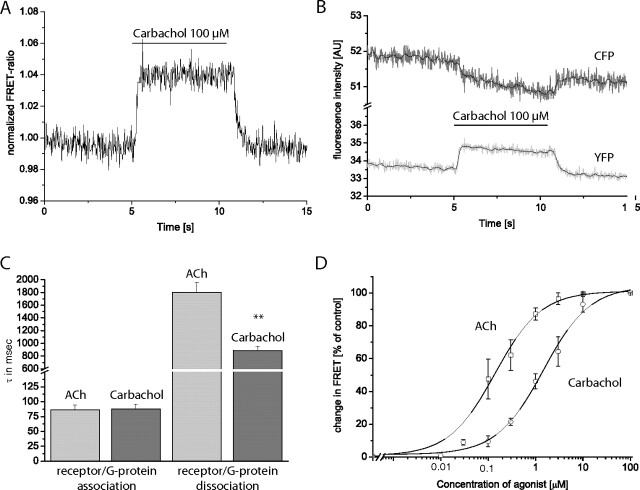
Fig. 3.
Analysis of receptor-G-protein interaction by FRET. FRET was measured between M3-AChR-YFP and CFP-tagged Gγ2. Excitation of CFP at 436 nm shifts the emission from 480 to 535 nm when the two fluorophores are close enough to permit FRET. A, HEK293 cells transiently expressing M3-AChR-YFP together with CFP-Gγ2 (and Gαq and β1) were superfused with 100 μM carbachol. This resulted in a rapid increase in FRET, as seen by the increase in the normalized FRET ratio (FYFP/FCFP). The increase in FRET was readily reversible upon agonist washout. B, upon superfusion with 100 μM carbachol, a decrease in CFP fluorescence and an increase in YFP fluorescence was observed. C, maximal receptor-G-protein interaction kinetics were compared with receptor-G-protein dissociation kinetics. Deactivation kinetics were analyzed similarly to activation kinetics. C, data for ACh (light gray) and carbachol (dark gray) for M3-AChR-YFP (data represent means ± S.E. values; n = 9 for each column). D, concentration-response curves for the M3-ACh receptor-G-protein interaction quantified by the amplitude of the ligand-evoked FRET changes. For ACh (□) EC50 values were 0.14 ± 0.02 μM, whereas for carbachol (○) EC50 values were 1.46 ± 0.26 μM, respectively (means ± S.E.; n = 10–12 for each ligand concentration). **, p < 0.01 (independent t test). AU, arbitary units.