TABLE 1.
Inhibition of PA-Nter by different DKA or DKA-bioisosteric compounds as determined in the plasmid-based enzymatic assay
One microgram of PA-Nter was incubated with 1 μg of M13mp18 plasmid substrate and the compounds. After 2 hours incubation, cleavage was assessed by gel electrophoresis.
| Scaffold | Compound | R 1 | R 2 | IC50a |
|---|---|---|---|---|
| μM | ||||
Piperidine scaffold 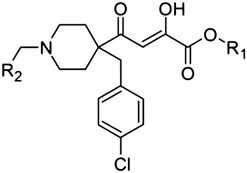
|
1 (L-742,001) | H | Ph | 0.5 |
| 2 | Me | Ph | 0.5 | |
| 3 | H | p-F-Ph | 1.4 | |
| 4 | Me | p-F-Ph | 0.4 | |
| 5 | H | Cy | 0.5 | |
| 6 | Me | Cy | 0.6 | |
Dihydroxybutenedioic acid 
|
7 | — | — | >500 |
1,3-Dioxo-1-phenylpropane scaffold and triazole bioisostere 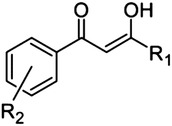
|
8 |
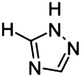
|
H | 77 |
| 9 | Ph | o-OH | >500 | |
Pyrrole scaffold 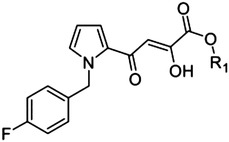
|
10 (L-731,988) | H | — | 0.8 |
| 11 | Me | — | 5.5 | |
2,4-Dioxo-4-phenylbutanoic acid (DPBA) scaffold 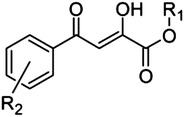
|
12 | H | H | 2.7 |
| 13 | Me | H | 14 | |
| 14 | H | o-OMe | 2.2 | |
| 15 | Me | o-OMe | 11 | |
| 16 | H | p-OMe | 2.0 | |
| 17 | H | p-Cl | 9.8 | |
| 18 | H | o-Cl | 9.8 | |
| 19 | Me | o-Cl | 15 | |
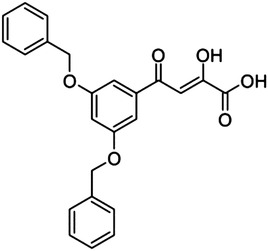
|
20 (L-708,906) | — | — | 137 |
2,4,6-Trioxo-6-phenylhexanoic acid scaffold 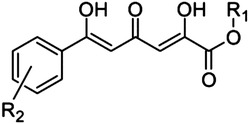
|
21 | H | H | >500 |
| 22 | Me | H | >500 | |
| 23 | H | o-OMe | >500 | |
| 24 | H | o-Cl | >500 | |
Quinolone scaffold 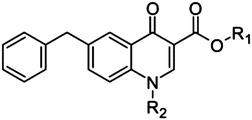
|
25 | H | H | >500 |
| 26 | H | CH2CH2OH | >500 | |
| 27 | H | Bn | >500 | |
| 28 | Et | Bn | >500 | |
Hydroxypyrimidine- carboxamide scaffold 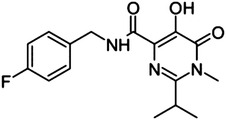
|
29 | — | — | >500 |
IC50, 50% inhibitory concentration. For each compound the IC50 value was calculated using nonlinear regression analysis. Values are the mean of at least three independent experiments.
