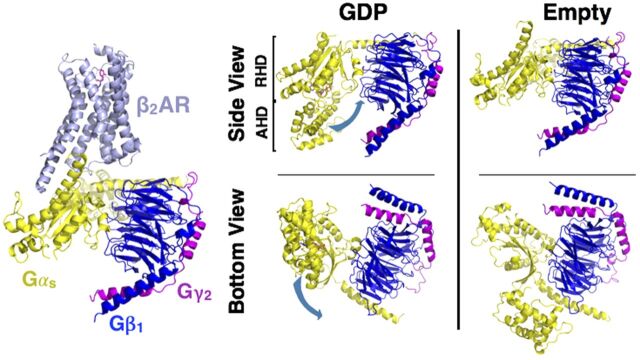
Fig. 8.
Structure of the Gsαβγ•β2-AR complex. Structure of the nucleotide-free Gsαβγ bound to agonist (Bi 1670107) in a complex with β2-AR (PDB: 3SN6). Structure of the GDP-bound heterotrimer of Gi (left, PDB: 1GP2) compared with the nucleotide-free form of Gs heterotrimer (far right panel, receptor excluded) reveals the conformational flexibility of the Gα subunit. Loss of GDP results in a large, rigid body translation (indicated by the blue arrow) of the α-helical domain (AHD) away from the ras homology domain (RHD) (right four panels).