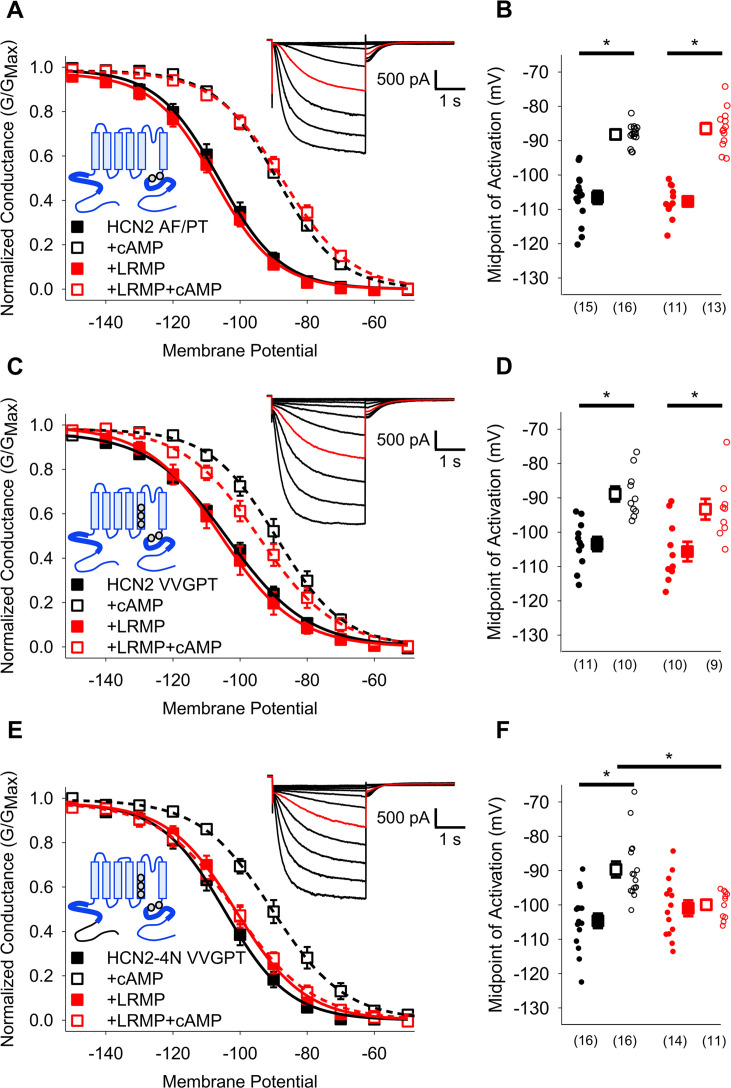
Figure 7. HCN4-specific residues and the HCN4 N-terminus confer LRMP regulation on HCN2.
(A) Voltage-dependence of activation for HCN2 A467P/F469T (AF/PT) in the absence (black) or presence of LRMP (red) and/or 1 mM intracellular cAMP (open symbols). (B) Average (± standard error of the mean) midpoints of activation for HCN2 AF/PT in the absence or presence of LRMP and/or 1 mM cAMP using the same color scheme as (A). (C) Voltage-dependence of activation for HCN2 VVGPT (M338V/C341V/S345G/A467P/F469T) in the absence or presence of LRMP and/or 1 mM intracellular cAMP using the same color scheme as (A). (D) Average (± standard error of the mean) midpoints of activation for HCN2 VVGPT in the absence or presence of LRMP and/or 1 mM cAMP using the same color scheme as (A). (E) Voltage-dependence of activation for HCN2-4N VVGPT (HCN4 1–212+HCN2 135-863 M338V/C341V/S345G/A467P/F469T) in the absence or presence of LRMP and/or 1 mM intracellular cAMP using the same color scheme as (A). (F) Average (± standard error of the mean) midpoints of activation for HCN2-4N VVGPT in the absence or presence of LRMP and/or 1 mM cAMP using the same color scheme as (A). Sample current insets: Exemplar current recordings for HCN2 AF/PT (A), HCN2 VVGPT (C), and HCN2-4N VVGPT (E) in the absence of LRMP and cAMP. Currents recorded with a –110 mV activating pulse are shown in red. Schematic Insets: Schematics of the chimeric channels with HCN4 sequence shown in black and HCN2 in blue. The HCN and cyclic-nucleotide binding domains are indicated as thicker line segments. Small circles represent individual recordings in (B, D) and (F) and values in parentheses are the number of independent recordings for each condition. * indicates a significant (p<0.05) difference. All means, standard errors, and exact p-values are in Table 2.