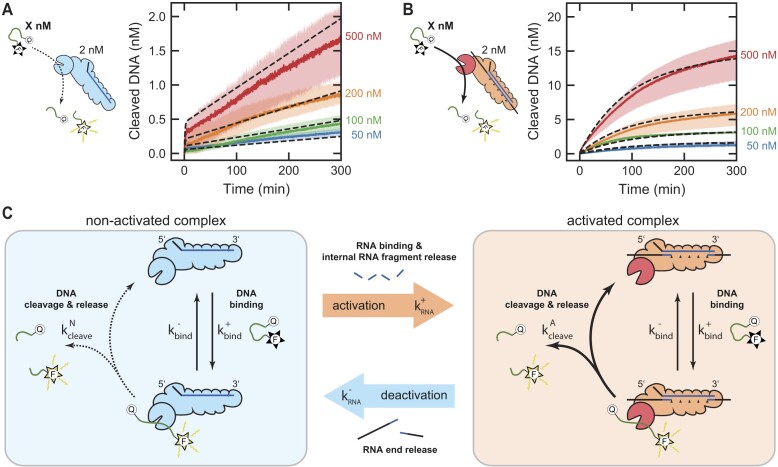
Figure 6.
A simple kinetic model for the RNA-dependent DNase activity of the Csm complex. (A, B) Bulk fluorescence measurements displaying the single-stranded DNase activity of the Csm complex across a range of ssDNA concentrations. The measurements were conducted using 2 nM Csm in the presence of 2 nM activating target RNA (B) and in its absence (A). Solid lines indicate the mean of three repeated measurements, while colored areas represent minimum and maximum values. Additionally shown is a global fit (dashed lines) of a simplified reaction scheme (see Supplementary Note S2). (C) Reaction scheme depicting the RNA-dependent DNase activity of the Csm complex. Both, the non-activated complex (not bound to any RNA fragments) and the activated complex (bound to RNA fragments), share the same single-stranded DNA binding kinetics, while differing in DNA cleavage activity. The single-stranded DNA initially binds to both complex entities in a reversible step and is subsequently cleaved and released in an irreversible step. Releasing the cleaved DNA enables the complex to bind new DNA molecules. The complex is activated by binding and cleavage of target RNA molecules. During activation, the short internal RNA fragments are rapidly released. Upon the release of the long RNA end fragments, the complex is deactivated.