Figure 7.
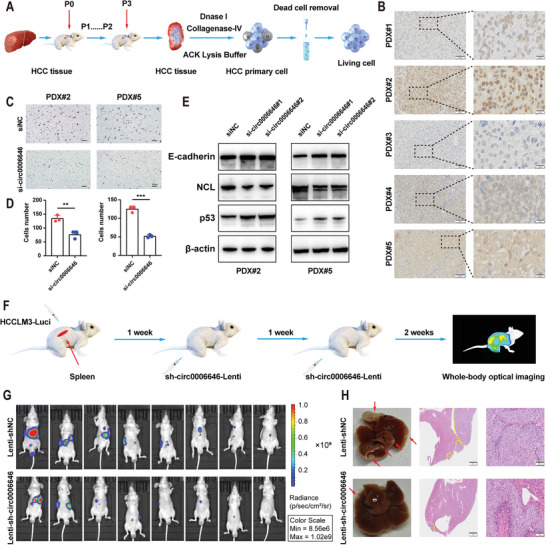
Circ0006646 might be a potential target for the treatment of HCC metastasis. A) The extraction process of primary HCC cells was presented. After 3 successive generations of HCC patient tumor tissue were cultured in mice, nucleic acid, erythrocyte, and extracellular matrix were digested with deoxyribonuclease, ACK lysing buffer, and collagenase‐IV, respectively. Then, the dead cells were removed, and the living primary HCC cells were obtained. B) ISH staining of circ0006646 was performed on 5 PDX models. Scale bars, 100 and 20 µm, respectively. C,D) After 2 primary HCC cells were transfected with siNC and si‐circ0006646, transwell assay was used to evaluate the migration and invasion ability. Scale bars, 50 µm. E) Two primary HCC cells were transfected with siNC and si‐circ000664. Blots with antibodies recognizing the E cadherin, NCL, p53, and β‐Actin were shown. F) Schematic diagram of simulated systemic therapy for HCC patients. The spleens of mice were injected with lucifera‐labeled HCCLM3 to construct liver metastasis model in vivo. shNC or sh‐circ0006646 lentivirus was injected through tail veins at the second and third weeks, followed by in vivo fluorescence imaging at the fourth week. G) Liver metastases were evaluated by in vivo fluorescence imaging, n = 8 mice/group. H) Representative photographs of liver showed the metastases (red arrows). HE staining was used to confirm the presence of metastases. Scale bars, 2 mm and 100 µm, respectively. All experiments were performed in at least triplicate samples. Data were presented as the means ± SD. * p < 0.05, ** p < 0.01, *** p < 0.001. Student's t‐test was used for normally distributed data and Mann–Whitney U test was used for skewed distribution data.
