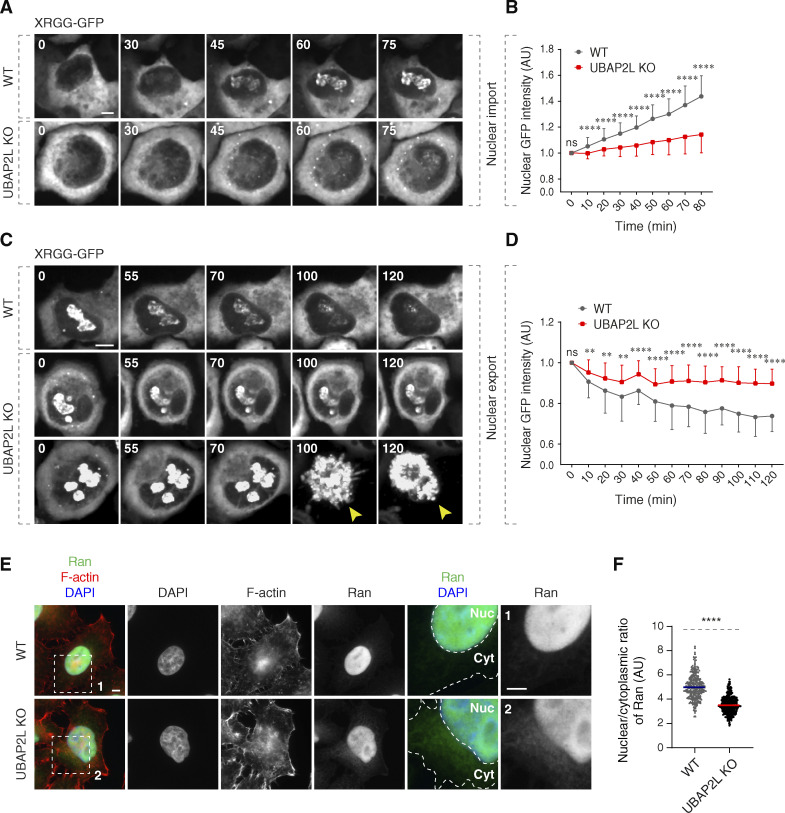
Figure 8.
UBAP2L regulates nucleocytoplasmic transport. (A–D) WT and UBAP2L KO HeLa cells expressing reporter plasmid XRGG-GFP for 30 h were analyzed by live video spinning disk confocal microscopy. The selected representative frames of the movies are depicted, and time is shown in minutes. Timepoint 0 in the top panel (nuclear import of XRGG-GPF) indicates that dexamethasone (0.01 μM) was added, while timepoint 0 in the bottom panel (nuclear export of XRGG-GPF) indicates that dexamethasone was washed out. The arrowheads indicate dead cells in UBAP2L KO cells. Scale bars, 5 μm (A and C). The nuclear intensity (fold change) of XRGG-GFP (to DNA labeled by SiR-DNA probe) in top panel (nuclear import) (B) and in bottom panel (nuclear export) (D) shown in A and C were quantified. At least 10 cells per condition were analyzed (mean ± SD, ns: not significant, **P < 0.01, ****P < 0.0001, unpaired two-tailed t test, n = 3 independent experiments). (E and F) Representative immunofluorescence images depicting the nuclear (Nuc) and cytoplasmic (Cyt) localization of Ran in asynchronously proliferating WT and UBAP2L KO HeLa cells. Actin filaments (also known as F-actin) were stained with phalloidin. The magnified framed regions are shown in the corresponding numbered panels. Nuclei were stained with DAPI. Scale bar, 5 μm (E). The N/C ratio of Ran shown in E was quantified (F) (mean ± SD, ****P < 0.0001, unpaired two-tailed t test; counted 277 cells for WT and 306 cells for UBAP2L KO).