Fig. 1.
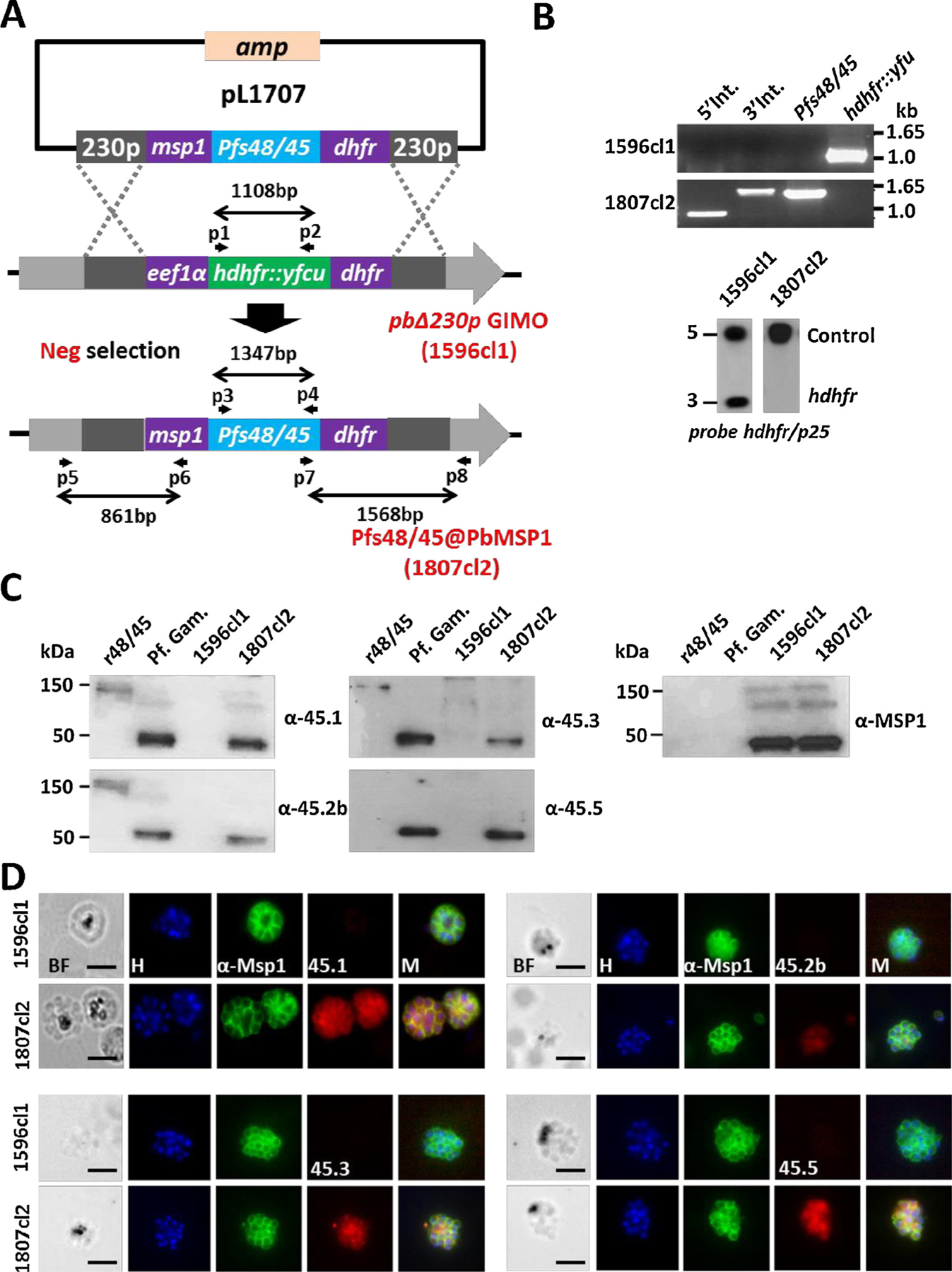
Generation, genotype and phenotype analyses of Pfs48/45@PbMSP1, a transgenic P. berghei parasite expressing P. falciparum P48/45 in schizonts.
(A) Schematic representation of the introduction of the Pfs48/45-expression cassette into the GIMOpbANKA parasite (line 1596cl1). Construct pL1707 contains the Pfs48/45 gene flanked by the msp1 promoter region and the 3′ pbdhfr UTR. This construct is integrated into the modified P. berghei 230p locus of GIMOpbANKA that contains the hdhfr::yfcu selectable marker (SM) cassette by double cross-over homologous recombination at the homology regions (230p; grey boxes). Negative selection with 5-FC selects for parasites that have the SM cassette replaced by the Pfs48/45 expression cassette. Location of primers used for PCR analysis and sizes of PCR products are shown.
(B) Diagnostic PCR (upper panel) and Southern analysis of PFG-separated chromosomes (lower panel) confirm correct integration of construct pL1707 in line 1807cl2 parasites. PCR shows the absence of the hdhfr::yfcu marker and the presence of the Pfs48/45. 5′ integration PCR (5′ int; primers p5/p6), 3′ integration PCR (3′ int; primers p7/p8), hdhfr::yfcu (primers p1/p2), Pfs48/45 (primers p3/p4). Primer locations and product sizes are shown in A and primer sequences in Table S1). Hybridization of PFG-separated chromosomes with a mixture of two probes (the hdhfr probe and a control probe recognizing p25 gene on chromosome 5) shows the removal of the SM cassette marker in the 230p locus on chromosome 3 in 1807cl2 parasites.
(C) Western analysis of Pfs48/45 expression in protein extracts of purified gametocytes of P. falciparum (Pf Gam), purified schizonts of wild type P. berghei (1596cl1) and purified schizonts of Pfs48/45@PbMSP1 (1807cl2). As a positive control, recombinant P. falciparum P48/45 fragment fused to GLURP R0 domain (R0.10C) was included (expected molecular size is 150 kDa). Blots were stained with 4 different anti-Pfs48/45 antibodies (45.1–3, 45.5) that recognize different epitopes. Anti-PyMSP1 antibody staining was used as a loading control.
(D) Immuno-fluorescence analyses of Pfs48/45 expression in purified schizonts of Pfs48/45@PbMSP1 (1807cl2), and the reference parent P. berghei GIMO line (i.e. WT; 1596cl1). Fixed parasites were stained with four different rat anti-Pfs48/45 mAbs (45.1–3, 45.5) and rabbit anti-PyMSP1 antibody followed by secondary conjugated antibodies anti-rabbit IgG Alexa Fluor ® 488 (green) or anti-rat IgG Alexa Fluor ® 594 (red). Nuclei stained with the DNA-specific dye Hoechst 33,342 (H). All pictures were recorded with the same exposure/gain times; anti-rabbit IgG Alexa Fluor ® 488 (green) 0.7 s; anti-rat IgG Alexa Fluor ® 594 (red) 0.6 s; Hoechst (blue) 0.136 s; bright field 0.62 s (1x gain). BF: bright field; M: merged. Scale bar: 2 μm.
