Figure 1.
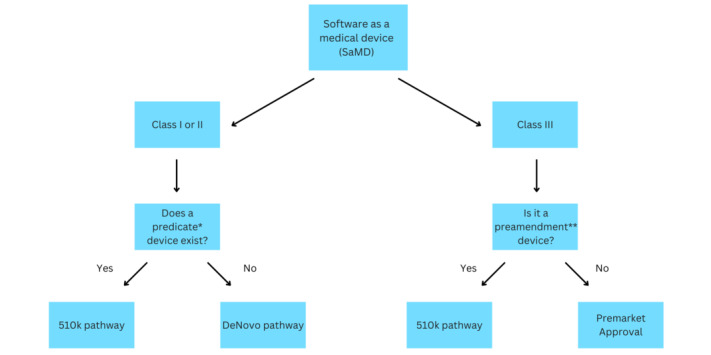
The current Food and Drug Administration (FDA) regulatory pathway. *A predicate: if the algorithm is found to be substantially equivalent to a legally marketed device. **A preamendment device: devices legally marketed in the United States before May 28, 1976, which have not been significantly changed/modified and for which no regulation requiring premarket approval has been published by the FDA.
