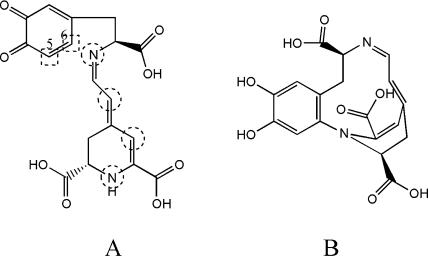
Figure 6.
Dopaxanthin-quinone evolution to more stable species implies intramolecular nucleophilic cyclization leading to structures similar to leuko-DOPA-chrome. A, Sites susceptible to giving internal cyclization are circled and the active positions of the ring are shown in squares. B, Suggested structure of the product derived from the attack of the distant nitrogen on position 6.