Fig. 1. Design of peptides to inhibit TANGO1-cTAGE5 heterodimerization.
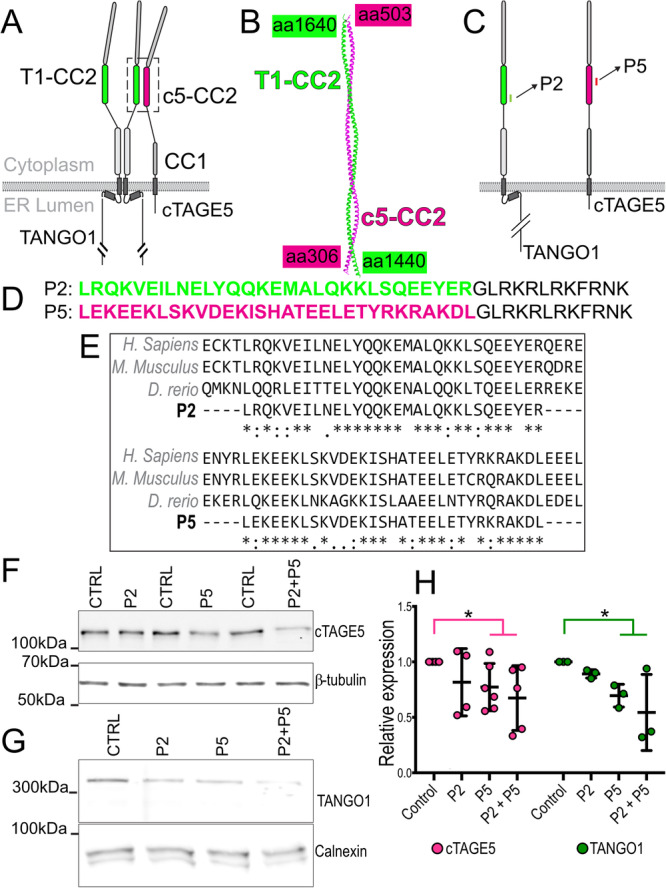
A The organization and the domains of TANGO1 and cTAGE5. Each of these proteins contains two cytoplasmic coiled-coil domains. TANGO1 coiled-coil 2 (T1-CC2) is highlighted in green. cTAGE5 CC2 in magenta. B Alphafold2 prediction of the structure of the heterodimer of TANGO1 (green) and cTAGE5 (magenta). C Schematic indicating where the peptide sequences map to in TANGO1 and cTAGE5. D Two peptides were synthesized of 30 (P2) and 31 (P5) amino acids respectively, conjugated to a 12 amino-acid C-terminal lysosomal escape motif (shown in black text). E Alignment of human, mouse, and zebrafish sequences. F Representative western blot of U2OS cell lysates treated with P2, P5 or P2 + P5, probed for cTAGE5. β-tubulin was used as a loading control. G Representative Western blot of U2OS cell lysates treated with P2, P5 or P2 + P5, probed for TANGO1. Calnexin was used as a loading control. H Quantification of blots showing TANGO1 (green) and cTAGE5 (magenta) levels (mean +/- SD) in control or peptide-treated cells (normalized to control) *p < 0.05, Student’s t test comparing cTAGE5 (magenta line) or TANGO1 (green line) in control vs treated. N = 3 (TANGO1), N = 5 (cTAGE5).
