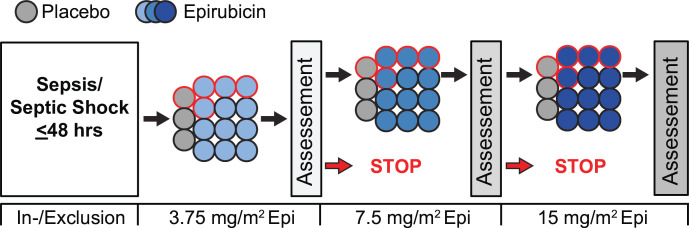
Figure 1.
Study design of the EPOS-1 trial. Black-bordered circles indicate minimal participants for the safety analysis. Red-bordered circles indicate patients who were randomised and received the study drug or placebo are expected not to reach the 14-day safety endpoint considering sepsis-related mortality of up to 30%. The assessment indicates a safety assessment of the data and safety monitoring board and a study continuation or stops following their recommendation. Epi, epirubicin; EPOS-1, Epirubicin for the Treatment of Sepsis and Septic Shock-1.