Figure 1.
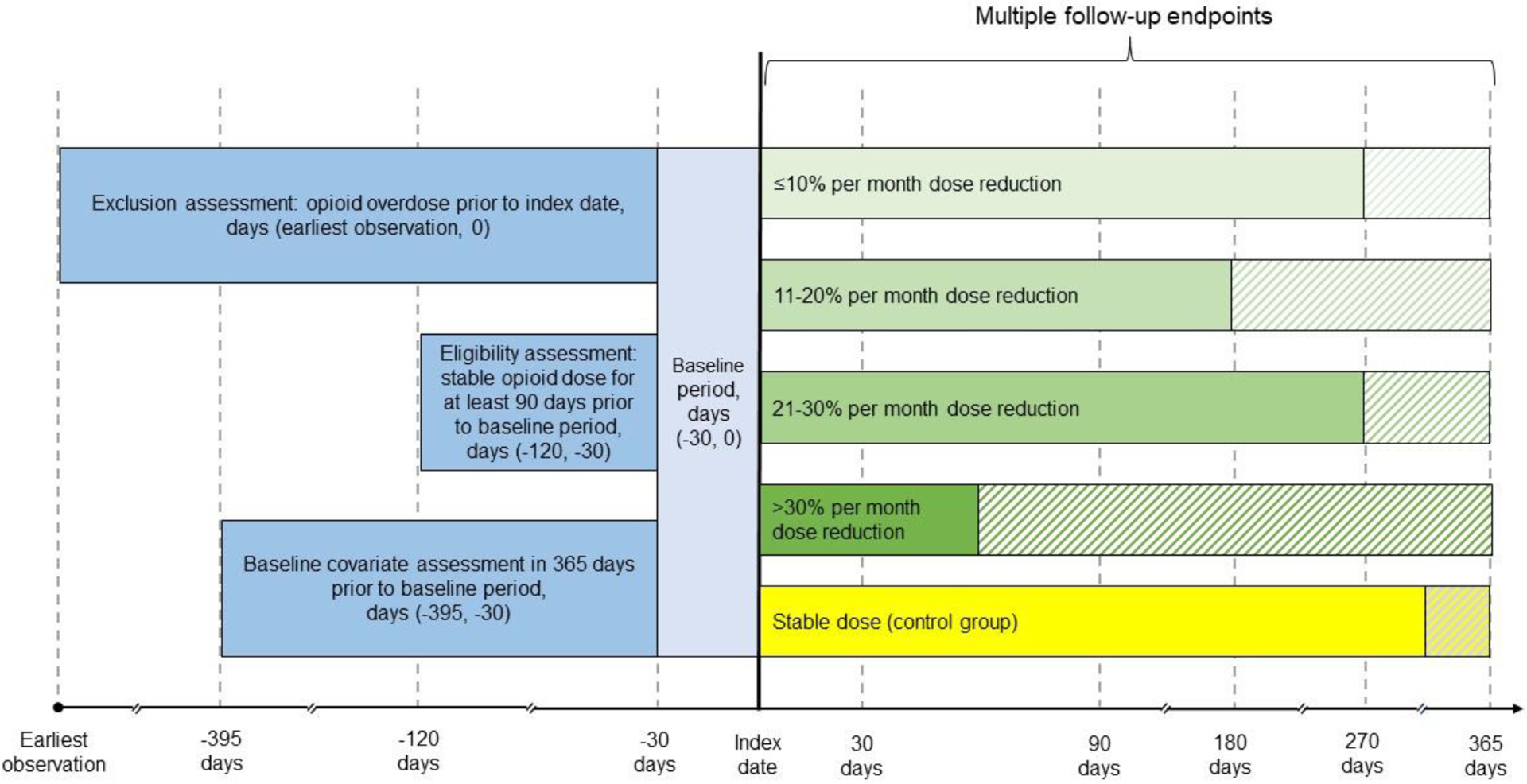
Schematic Diagram of the Study Designa
aA baseline period of 30 days was needed to determine opioid dose patterns. Patients were required to have at least 90 days of stable dosing prior to the beginning of the baseline period to be eligible for inclusion in the analysis. Patients with one or more opioid overdoses during the baseline period were excluded from the analysis. Baseline covariates were assessed in the year before the baseline period. Dose patterns are shown with varying lengths of follow-up time to illustrate variability in duration. The shaded areas represent follow-up after the index dose pattern. Patients may experience any dose pattern(s) after the index dose, except for a dose reduction preceded by ≥3 months of a stable dose (i.e., the qualifying condition to be included in the exposure group) in the control group.
