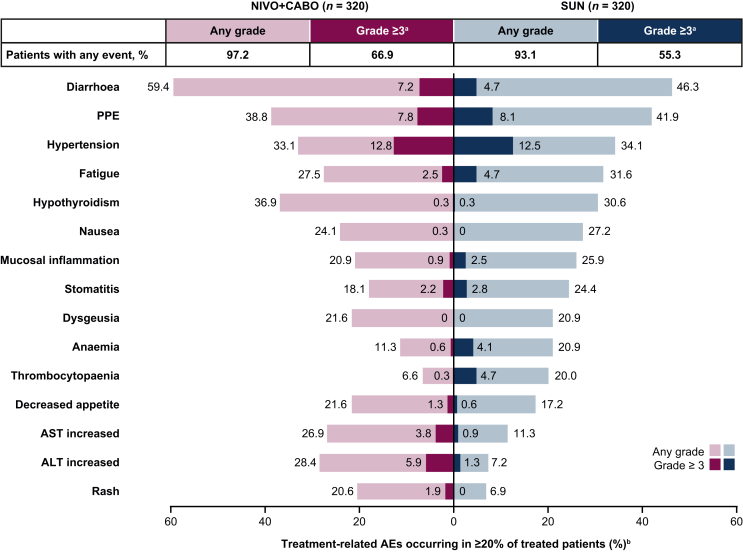
Figure 3.
Treatment-related AEs in ≥20% of all treated patients. AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; NIVO + CABO, nivolumab plus cabozantinib; PPE; palmar–plantar erythrodysaesthesia; SUN, sunitinib.aNo patients had a grade 5 event with NIVO + CABO and one patient had a grade 5 event with SUN. bTotal bar represents treatment-related AEs of any grade in either treatment arm reported between first dose and 30 days after last dose of study therapy; of these events, none were grade 5.