Fig. 3.
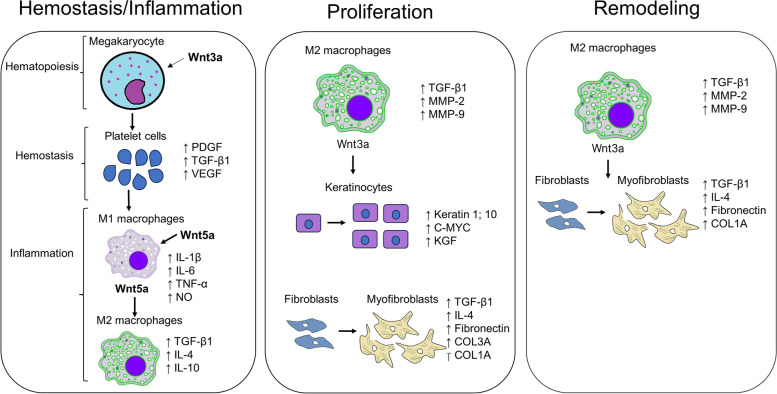
The involvement of Wnt/β-catenin signaling in wound healing. During hemostasis and inflammation, Wnt3a is known to induce the formation of proplatelet cells that mature into platelet cells and lead to the increased production and secretion of platelet-derived growth factor (PDGF), transforming growth factor-beta 1 (TGF-β1) and vascular endothelial growth factor (VEGF). The secreted growth factors from platelet cells also activate circulating and bone-marrow derived monocytes to proliferate and migrate to the wound site and differentiate into M1 macrophages which express and secrete interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α), nitric oxide (NO), as well as Wnt5a, which further activates transdifferentiation of M1 macrophages into anti-inflammatory M2 macrophages. M2 macrophages produce and secrete anti-inflammatory cytokines (TGF-β1; IL-4; IL-10) that leading to the progression to the proliferative phase, where the TGF-β1 and Wnt3a secreted by M2 macrophages activate the proliferation and migration of keratinocytes and fibroblasts for formation of granulation tissue. M2 macrophages also secrete metalloproteinase-2 (MMP-2) and MMP-9 for degradation of old ECM and deposition of new ECM. At the proliferative phase fibroblasts also transdifferentiate into contractile myofibroblasts expressing alpha-smooth muscle actin (α-SMA), fibronectin as well as collagen type 3A1 (COL3A1) and (COL1A1). At the remodeling phase, the activated M2 macrophages and myofibroblasts further secrete TGF-β1 and Wnt3a for further proliferation and differentiation of fibroblasts into myofibroblasts for wound contraction and deposition of COL1A which is involved in scar formation