Fig. 3. Analysis of three compounds selected from the structural similarity search for effects on RORγ activity.
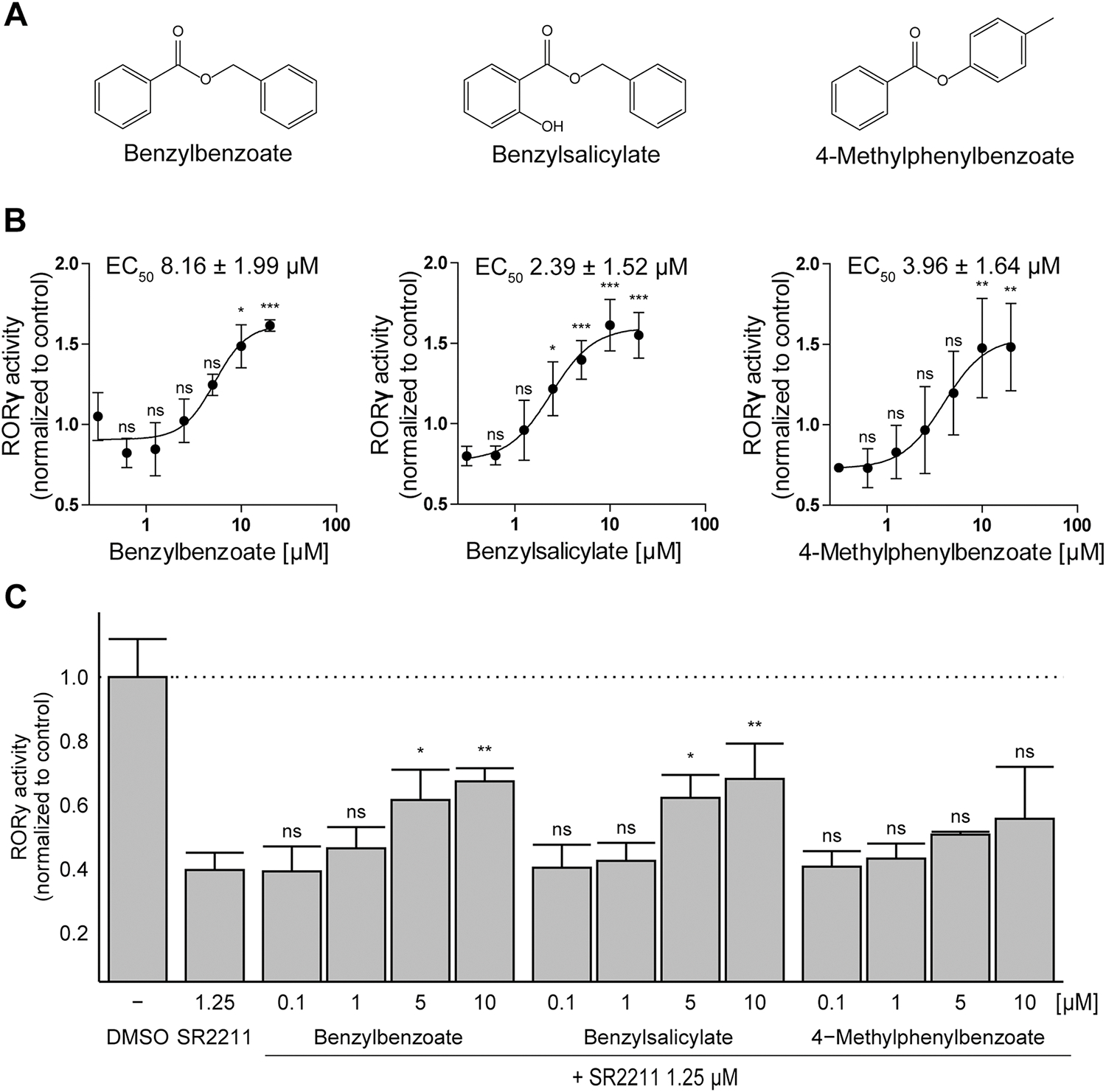
(A) Chemical structures of the three RORγ activators identified by structural similarity search. (B) Concentration-dependent activation of RORγ by the selected compounds. Concentration-response curves were fitted and analyzed by non-linear regression. (C) Activation of RORγ by the three selected compounds in the presence of a RORγ inverse agonist. RORγ expression was induced by doxycycline in the absence (B) or presence (C) of 1.25 μM SR2211. Cells were incubated with the test compounds at the concentrations indicated. Luciferase reporter activity was determined and normalized to that of the DMSO vehicle control. Data from three independent experiments represent mean ± SD. Data were analyzed by one-way ANOVA with Dunnett’s post-hoc test, p values: * < 0.05, ** < 0.01, and ns (not significant).
