Abstract
Recent evidence suggests that the gut microbiota plays a role in insomnia pathogenesis. This study compared the dietary habits and microbiota metabolites of older adults with insomnia of short vs. normal sleep duration (ISSD and INSD, respectively). Data collection included sleep assessment through actigraphy, dietary analysis using the Food Frequency Questionnaire, and metabolomic profiling of stool samples. The results show that ISSD individuals had higher body mass index and a greater prevalence of hypertension. Significant dietary differences were observed, with the normal sleep group consuming more kilocalories per day and specific aromatic amino acids (AAAs) phenylalanine and tyrosine and branch-chain amino acid (BCAA) valine per protein content than the short sleep group. Moreover, metabolomic analysis identified elevated levels of the eight microbiota metabolites, benzophenone, pyrogallol, 5-aminopental, butyl acrylate, kojic acid, deoxycholic acid (DCA), trans-anethole, and 5-carboxyvanillic acid, in the short compared to the normal sleep group. The study contributes to the understanding of the potential role of dietary and microbial factors in insomnia, particularly in the context of sleep duration, and opens avenues for targeted dietary interventions and gut microbiota modulation as potential therapeutic approaches for treating insomnia.
Keywords: amino acids, deoxycholic acid, diet, insomnia, microbiota metabolites, sleep duration
1. Introduction
Insomnia is a common chronic health condition and the most common sleep disorder, with symptoms affecting approximately 50% of adults over the age of 65 [1]. Insomnia, as defined by the Diagnostic and Statistical Manual of Mental Disorders, 5th edition (DSM-5) [2], is a sleep–wake disorder characterized by dissatisfaction with sleep quality or quantity. It involves difficulty initiating or maintaining sleep, early morning awakenings, nonrestorative sleep and significant distress or impairment in various areas of life [2,3,4]. The symptoms must occur for at least three nights per week for a duration of at least three months, and should not be better explained by another sleep, mental, or medical disorder [1]. Insomnia in the older population is linked to a range of disease risks such as diabetes, metabolic syndrome, cognitive impairment, and depression, imposing high costs on healthcare systems and society [5].
Evidence supports a distinction between two phenotypes of insomnia defined based on a short vs. normal sleep duration. Insomnia with short sleep duration (ISSD), typically defined as a total sleep time of ≤6 h [6,7], is the most biologically severe phenotype and is associated with physiological hyperarousal and increased risk of cardiometabolic morbidity and mortality [6,8,9]. In contrast, insomnia of normal sleep duration (INSD) (typically defined as a total sleep time of >6 h) is associated with an overall lower risk of cardiometabolic outcomes compared to ISSD [6,7]. Moreover, INSD individuals are characterized by cognitive–emotional and cortical arousal. Because their insomnia is more closely linked to cognitive and emotional factors rather than physiological ones, these individuals are likely to respond better to Cognitive Behavioral Therapy for Insomnia (CBT-I) [10].
Growing evidence shows that the microbiota gut–brain axis contributes to the regulation of sleep behavior both directly and indirectly and may play a critical role in the etiology and pathogenesis of sleep disorders [11]. It has been shown that in older people with insomnia, differences in the composition of the gut microbiota and an abundance of particular genera, such as Lactobacillus crispatus and Streptococcus, were found to be associated with poor sleep and poor cognitive function [12]. Five metabolic pathways, including those for glycerophospholipid metabolism, glutathione metabolism, nitrogen metabolism, aspartate, glutamate, alanine metabolism, and aminoacyl-tRNA production, may be involved in the link between the gut microbiota and insomnia [13].
The gut microbiota communicates with the brain through the microbiota gut–brain axis (MGBA) [14]. The MGBA comprises neuronal, immune, metabolic, and endocrine pathways [11,15]. Neurotransmitters and metabolites produced by gut microbes, such as gamma-aminobutyric acid (GABA), dopamine, and serotonin (5-HT), can affect neurons of the enteric nervous system (ENS) and interact with afferent pathways of the vagal nerve, affecting the neural circuits involved in sleep–wake regulation [16]. Similarly, gut-derived metabolites can be transmitted to the brain through blood circulation and afferent vagal pathways to affect sleep [17]. Indeed, alterations in the gut microbiota composition have been correlated with specific serum metabolites [18,19], sleep quality and cognitive performance [20] in patients with insomnia.
Gut microbiota metabolites originate from the breakdown of diet nutrients, mainly diet fibers. Proteins, on the other hand, are mostly digested and absorbed in the small intestine. Proteins that escape digestion reach the colonic lumen, where they serve as fermentable substrates for the gut microbiota and undergo proteolysis into amino acids. Colonic amino acids may be further metabolized by the gut microbiota, as exemplified by aromatic amino acids (AAAs) [21]. AAA catabolism by the gut microbiome yields numerous metabolites that can regulate immune, metabolic, and neuronal responses at local and distant sites. Phenylalanine (Phe) is a metabolic precursor of tyrosine (Tyr) via phenylalanine hydroxylase in the liver, which further metabolizes to catecholamines (dopamine, norepinephrine and epinephrine) [22].
Other key metabolites produced by the gut microbiota include secondary bile acids (BAs). The primary BAs pool in humans consists of cholic acid, chenodeoxycholic acid, and subsequent C24 taurine- or glycine-bound derivatives, synthesized in the liver from cholesterol [23]. Primary BAs are heavily modified in the lower gastrointestinal tract to produce a broad range of secondary BAs [23]. Abnormally high levels of the microbially modified secondary BA deoxycholic acid (DCA) are associated with gut dysbiosis and disease [24,25].
Although research on the involvement of gut microbiota metabolites is mounting, the possible involvement of gut metabolites in sleep regulation and insomnia remains largely unknown. Previous studies have shown that individuals with insomnia exhibit distinct metabolic profiles compared to matched controls, including alterations in branched-chain amino acid (BCAA) metabolism and lactate peak timing and amplitudes [1]. It is critical to note that while these studies offer valuable insights into the relationship between sleep and metabolism, they focus predominantly on metabolomic analysis of blood samples rather than fecal samples. This study compared the dietary intake and fecal metabolite profiles of individuals with short vs. normal sleep duration insomnia. As ISSD is essentially a more physiological phenotype of the disorder, we hypothesized that gut metabolites associated with dysbiosis would be upregulated in short sleepers. In addition, we expected lower levels of vital dietary nutrients known to influence sleep in older adults with ISSD.
2. Materials and Methods
The study protocol was approved by the institutional review board (IRB) of the Faculty of Social Welfare and Health Sciences at the University of Haifa (approval number 026/17). All participants involved in the study provided informed consent.
2.1. Study Cohort
In this study, a focused cohort of 25 participants was derived from a broader preliminary study involving 59 participants [26]. Participants were categorized into two different types of insomnia, INSD and ISSD, using the SPSS K-means clustering analysis, based on two key indicators: sleep efficiency and sleep duration, as measured by actigraphy [26]. This subset was meticulously chosen based on stringent inclusion criteria to ensure a homogeneous sample that accurately reflects distinct insomnia sleep duration phenotypes, short vs. normal. The inclusion criteria for insomnia were sleep onset latency (SOL) or wake after sleep onset (WASO) of >30 min; and less than 85% sleep efficiency (SE, percentage of total sleep time after initial sleep onset out of total time in bed) for at least three out of seven nights each week [26]. The exclusion criteria were chronic pain; substantial and unstable medical, neurological, or psychiatric illness; any significant visual or hearing impairments; psychiatric medication use; alcohol or drug use; sleep apnea syndrome (SAS); and periodic limb movement disorder during sleep (PLMD), based on a self-report. Participants were tested on the Mini Mental State Examination (MMSE) [27] using a cutoff >26 to exclude participants with cognitive impairment.
2.2. Sleep Assessment
Sleep was assessed through a clinical interview conducted by telephone by a trained interviewer. Participants were asked about medical condition(s), medication(s), or other substance use, and responded to questions about specific nighttime sleep problems such as frequency and duration of insomnia and difficulties falling asleep or staying asleep. In addition, participants were instructed to wear an Actiwatch, an activity wrist monitor, for a period of two weeks, and total sleep time (TST), SOL, SE, and WASO were collected.
2.3. Food Frequency Questionnaire
The dietary assessment was performed using the 127-item Food Frequency Questionnaire (FFQ). The development and validation process of this questionnaire is described in detail elsewhere [28]. Briefly, the FFQ includes 127 food items with nine frequency options ranging from “never or less than once monthly” to “six or more times daily”. The questionnaire is semi-quantitative, and a standard portion size is described for each food item. The portion-size estimates are based on information from the Israeli Ministry of Health. Participants are asked to report their average frequency of consumption during the past year.
2.4. Untargeted Metabolomics
Recruited individuals were asked to provide a morning stool sample, which was stored at −20 °C until analysis. A modified liquid chromatography–mass spectrometry (LCMS) analysis protocol [29] was used for stool sample preparation and analysis. In brief, samples were homogenized, frozen, dried, and then pulverized. The samples were then centrifuged, and the upper liquid was transferred for filtration and subsequent LC-MS analysis.
The samples were injected Into an UHPLC connected to a photodiode array detector (Dionex Ultimate 3000, (Thermo Fisher Scientific, San Diego, CA, USA) with a reverse-phase column (ZORBAX Eclipse Plus C18, 3.0 × 100 mm, 1.8 µm, Agilent Technologies, Santa Clara, CA, USA). MS/MS analysis was performed with a heated electrospray ionization source connected to a Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ Mass Spectrometer (Thermo Fisher Scientific, San Diego, CA, USA). The gradient was initiated with 2% B, which was increased to 30% B over a period of 4 min, and then increased to 40% B over 1 min before being kept isocratic at 40% B for another 3 min. Then, the gradient increased to 50% over 6 min, to 55% over another 4 min, and to 95% over 5 min, and was kept isocratic for 7 min. Finally, phase B was returned to 2% over 3 min and the column was allowed to equilibrate at 2% B for 3 min before the next injection. The flow rate was 0.4 mL/min. Blank (methanol) and QC samples were injected at the start of the sequence, after every 10 samples, and at the end of the sequence.
LC–MS/MS analysis was performed with a Heated Electrospray ionization (HESI-II) source connected to a Q Exactive™ Plus Hybrid Quadrupole-Orbitrap™ Mass Spectrometer, Thermo Scientific™, Dreieich, Hessen Germany. The ESI capillary voltage was set to 3500 V, the capillary temperature to 300 °C, gas temperature to 350 °C, and the gas flow to 10 mL/min. The mass spectra (m/z 100–1500) were acquired using both positive and negative ion modes. Data-dependent MS2 analysis was generated for the QC samples and used for compound identification. Downstream analysis and data processing were performed with the Thermo Scientific™ Compound Discoverer™ program, version 3.1.0.305 (mass tolerance ≤ 5 ppm; intensity tolerance ≤ 30%; S/N threshold = 3; minimum peak intensity = 1,000,000; RT tolerance ≤ 0.2 min). Databases used for identification were Chemspider [30], MzCloud [31] and KEGG [32].
2.5. Statistical Analysis
Statistical tests were employed to examine differences in the prevalence of metabolites, sleep measures, demographic characteristics, quality of life and dietary consumption between the short and normal groups. Data were processed and analyzed using various statistical tools including independent-samples t-tests, nonmetric dimensional scaling (NMDS), ANOSIM, MANOVA, and Mann–Whitney tests, using software such as Compound Discoverer (version 3.1) (Thermo Fisher Scientific, San Diego, CA, USA), R software (version 3.6.2) https://www.r-project.org/, SPSS software (version 20.0) (Add reference present in comment), and GraphPad Prism 8 software (version 8.3.1) https://www.graphpad.com.
3. Results
3.1. Demographic and Sleep Characteristics
The demographic and sleep characteristics of the study cohort are shown in Table 1. The average age of the entire study population was 74.9 ± 6.9 years, and most of the participants were female (91.7%). No significant differences were detected between the normal and short groups in terms of age, gender distribution, educational background, marital status or living status (alone or accompanied). The short sleepers, however, demonstrated a significantly higher body mass index (BMI) (33.91 ± 7.77 kg/m2) than normal sleepers (24.79 ± 4.18 kg/m2; p = 0.0011). In addition, short sleepers had a significantly higher prevalence of hypertension (77.8%) compared to the normal sleep group (20.0%) (Chi-squared p = 0.0053). The prevalence of diabetes and usage of medication for sleep or depression, as well as usage of anticholinergic medications, were similar across groups (Table 1).
Table 1.
Characteristics of the study population.
| Parameter | Study Population (n = 25) |
INSD (n = 15) |
ISSD (n = 10) |
p |
|---|---|---|---|---|
| Age (years) | 74.88 ± 6.94 | 74.2 ± 8.42 | 75.90 ± 4.01 | 0.560 1 |
| Gender (%) | ||||
| Female | 91.7 | 86.7 | 100 | 0.229 3 |
| Male | 8.3 | 13.3 | 0 | |
| Education (years) | 17.0 ± 2.0 | 17.0 ± 2.0 | 17.0 ± 3.0 | 0.892 2 |
| Marital status (%) | ||||
| Married | 64.0 | 60.0 | 66.7 | 0.744 3 |
| Other | 36.0 | 40.0 | 33.3 | |
| Living status (%) | ||||
| Lives with roommates | 64.0 | 60.0 | 66.7 | 0.744 3 |
| Lives alone | 36.0 | 40.0 | 33.3 | |
| BMI (kg/m2) | 28.59 ± 7.39 | 24.79 ± 4.18 | 33.91 ± 7.77 | 0.001 1 |
| Metabolic syndromes (%) | ||||
| Diabetes | 20.8 | 13.3 | 33.3 | 0.243 3 |
| Hypertension | 41.7 | 20 | 77.8 | 0.005 3 |
| Heart disease | 8.3 | 6.7 | 11.1 | 0.703 3 |
| Medication use (%) | ||||
| Sleep medications | 20 | 23.1 | 14.3 | 0.639 3 |
| Depression medications | 20 | 30.8 | 0 | 0.101 3 |
| Anticholinergic medications | 15 | 7.7 | 28.6 | 0.375 3 |
| Physical activity (%) | 91.7 | 100 | 77.8 | 0.057 3 |
| Screen time during free time (min) | ||||
| Television | 134.38 ± 78.81 | 130.00 ± 76.63 | 141.67 ± 86.53 | 0.734 1 |
| Computer | 87.08 ± 61.68 | 89.33 ± 57.51 | 83.33 ± 71.59 | 0.726 2 |
| Sleep measurements | ||||
| TST (min) | 403.84 ± 67.91 | 446.34 ± 41.85 | 340.08 ± 45.24 | <0.001 1 |
| SOL (min) | 17.81 ± 15.03 | 15.94 ±12.66 | 20.61 ± 18.41 | 0.723 2 |
| SE (%) | 82.16 ± 7.71 | 84.02 ± 4.04 | 79.37 ± 10.89 | 0.196 2 |
| ST (time of the day in decimal number) | 24.36 ± 1.33 | 23.79 ± 0.94 | 25.22 ± 1.40 | 0.005 1 |
| ET (time of the day in decimal number) | 7.00 ± 1.09 | 7.23 ± 0.94 | 6.67 ± 1.26 | 0.217 1 |
| WASO (min) | 49.31 ± 16.61 | 51.26 ± 14.22 | 46.39 ± 20.13 | 0.484 1 |
The values are presented in the table as mean ± standard deviation (mean ± SD), except the values of the variables gender, marital status, accommodation, metabolic syndromes, medication use, and physical activity, which are shown in frequency (%). p represents the level of significance of the differences between the normal group and the short group, as determined by 1 independent-samples t-test, 2 Mann–Whitney, 3 Chi-squared. TST—total sleep time; SOL—sleep onset latency; SE—sleep efficiency; ST—start time; ET—end time; WASO—wake after sleep onset; INSD = insomnia of normal sleep duration; ISSD = insomnia of short sleep duration. p < 0.05 are shown in bold.
Normal sleepers tended to be more physically active (100%) compared to short sleepers (77.8%; p = 0.0573). The television and computer screen time were similar between groups (Table 1).
TST and ST were the two key parameters used to define INSD and ISSD. No significant differences were noted for other sleep parameters, such as SOL, SE, ET, and WASO.
3.2. Significant Differences in Nutritional Intake between INSD and ISSD Participants
The mean participant nutritional intake of the two study groups is summarized in Table 2 and Supplementary Data S1. Out of the 25 participants included in this study, 19 completed the FFQ questionnaire (n = 11 and 8, normal and short group, respectively) and were included in the analysis. The participants in the normal group consumed significantly more kilocalories throughout the day than those in the short group (p = 0.023), while the relative consumption of proteins, carbohydrates, and fats (%/total kcal) was not significantly different between the groups (p > 0.05). Since ISSD had a significantly lower energy intake in comparison with the ISND group, lower levels of all macro and micronutrients were expected to be present in the ISSD group, creating bias. Therefore, we calculated the % of each dietary compound within its category (e.g., % amino acids/total protein), and performed a statistical analysis between both groups. The results show that, except for glutamic acid, proline, serine, hydroxyproline, leucine, isoleucine and tryptophan, the % of all amino acids/total protein was significantly higher in the normal group compared to the short group (p < 0.05). No differences in fatty acid, mineral, and vitamin percentages were detected between groups (Supplementary Data S1).
Table 2.
Nutritional intake of the participants with INSD vs. ISSD, as measured by the Food Frequency Questionnaire (FFQ).
| Nutrient | INSD (n = 11) |
ISSD (n = 8) |
p |
|---|---|---|---|
| Total Energy (kcal/day) | 2423.8 ± 693.1 | 1775.6 ± 434.9 | 0.023 |
| Macronutrients (%/kcal) | |||
| Proteins | 17.47 ± 2.68 | 17.57 ± 2.36 | 0.932 |
| Carbohydrates | 48.15 ± 7.34 | 48.07 ± 6.51 | 0.981 |
| Fats | 34.39 ± 6.81 | 34.36 ± 5.49 | 0.996 |
| Fibers (%/kcal) | 3.55 ± 0.54 | 3.65 ± 0.50 | 0.697 |
| Amino Acids (%/total protein) | |||
| Cystine | 2.05 ± 0.55 | 1.53 ± 0.43 | 0.035 |
| Phenylalanine | 7.27 ± 2.47 | 5.34 ± 1.21 | 0.040 |
| Tyrosine | 5.59 ± 2.06 | 3.93 ± 0.96 | 0.034 |
| Valine | 8.33 ± 2.91 | 5.87 ± 1.31 | 0.025 |
| Arginine | 8.52 ± 2.62 | 6.17 ± 1.50 | 0.025 |
| Histidine | 4.13 ± 1.44 | 2.97 ± 0.74 | 0.035 |
| Alanine | 7.22 ± 2.36 | 5.30 ± 1.29 | 0.037 |
| Aspartic acid | 14.32 ± 4.03 | 10.59 ± 2.27 | 0.020 |
| Glycine | 5.78 ± 1.67 | 4.34 ± 1.13 | 0.039 |
| Proline | 10.63 ± 4.48 | 7.43 ± 2.08 | 0.055 |
| Serine | 7.41 ± 2.56 | 5.28 ± 1.19 | 0.028 |
The table presents the values as mean ± standard deviation (mean ± SD). p represents the significance level of the differences between the normal group and the short group, as determined by independent-samples t-test. kcal = kilocalories; INSD = insomnia of normal sleep duration; ISSD = insomnia of short sleep duration. p < 0.05 are shown in bold.
3.3. LCMS Analysis
3.3.1. Distinct Metabolite Compounds Associated with INSD and ISSD Participants
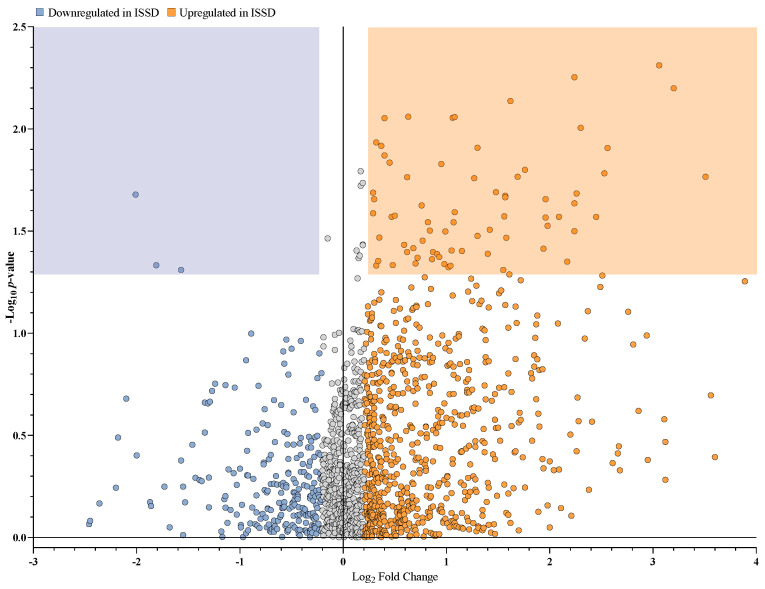
We conducted untargeted LCMS to analyze fecal samples collected from INSD and ISSD participants (n = 15 and n = 10, respectively). We found 19,502 compounds, of which 1451 were identified as different metabolites (with names and without isomers) (Supplementary Data S2). Among the 1451 metabolites identified, 82 were significantly upregulated, and 3 were significantly downregulated in the ISSD group (Figure 1).
Figure 1.
Upregulated and downregulated metabolites in fecal samples of ISSD participants. Each data point in the volcano plot represents a single metabolite, with the x-axis indicating the average relative change or fold change (in log2 scale) between downregulated (blue) and upregulated (orange) metabolites in ISSD participants. The y-axis represents the p-value (in −log10 scale) for the relative change for each metabolite. A Log2 fold change of less than −0.2 and a p-value of less than 0.05, are commonly accepted for statistical significance. The orange and blue areas represent metabolites that were significantly upregulated and downregulated in the ISSD group, respectively. Grey data points represent metabolites that were not significantly different. ISSD = Insomnia of short sleep duration.
3.3.2. Similar Metabolite Profile between INSD and ISSD Participants
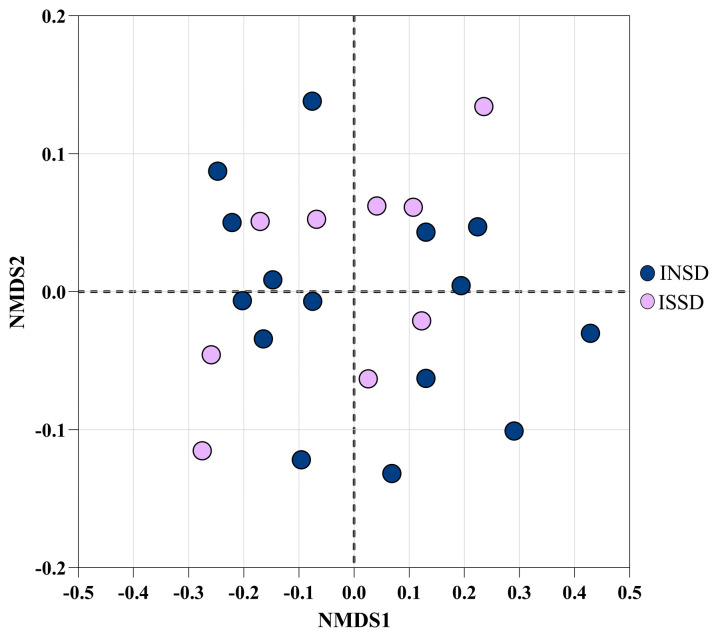
We performed a nonmetric multidimensional scaling (NMDS) of Bray–Curtis dissimilarity to test for metabolite dissimilarity between INSD and ISSD participants (Figure 2). The samples from the normal and short groups were found to be almost uniformly distributed across the center of the plot, suggesting that the metabolite profiles of these two groups were not markedly different. An ANOSIM statistical test confirmed the absence of a significant difference in the metabolite profile of the two study groups (p > 0.05).
Figure 2.
Nonmetric multidimensional scaling (NMDS) of Bray–Curtis dissimilarity (Î2 diversity) in INSD and ISSD participants. Dark blue and purple points represent INSD and ISSD participants, respectively.
3.3.3. Upregulated Metabolites Linked to Bacterial Metabolic Pathways in ISSD Participants
From the 82 metabolites found to be significantly upregulated in the ISSD group in comparison to the INSD group, eight (benzophenone, pyrogallol, 5-aminopental, butyl acrylate, kojic acid, deoxycholic acid (DCA), trans-anethole, and 5-carboxyvanillic acid) were linked to bacterial metabolic pathways (Table 3).
Table 3.
Bacterial metabolic pathway-associated metabolites significantly upregulated in ISSD participants.
| Metabolite Name | Formula | RT (min) | Log2 Fold Change | p-Value |
|---|---|---|---|---|
| Benzophenone | C13H10O | 16.841 | 3.23 | 0.001 |
| Pyrogallol | C6H6O3 | 1.765 | 0.63 | 0.009 |
| 5-aminopental | C5H11NO | 2.034 | 0.32 | 0.012 |
| Butyl acrylate | C7H12O2 | 8.525 | 0.45 | 0.015 |
| Kojic acid | C6H6O4 | 1.776 | 0.47 | 0.027 |
| Deoxycholic acid | C24H40O4 | 19.577 | 0.84 | 0.031 |
| trans-anethole | C10H12O | 19.625 | 0.87 | 0.040 |
| 5-carboxyvanillic acid | C9H8O6 | 4.575 | 1.55 | 0.049 |
p represents the significance level of the differences between the normal and short groups, as determined using the t-test. RT—retention time; Log2 fold change—the average relative change on a Log2 scale. The Log2 fold change column represents the relative difference between the groups on a Log2 scale, where a positive value indicates higher levels in the ISSD group compared to the ISND group.
4. Discussion
This study aimed to gain further insights into the possible contribution of microbiome metabolites to short or normal sleep duration phenotypes of insomnia in older adults. Significant differences were found in the BMI, blood pressure, amino acid consumption and gut microbiome metabolite profiles of individuals with short vs. normal sleep-duration insomnia. Our main findings are (i) older adults with ISSD consumed lower levels of amino acids such as phenylalanine, tyrosine, and valine, which are known to play a role in sleep regulation and (ii) DCA, a gut metabolite associated with dysbiosis and disease, was upregulated in ISSD older adults. Overall, these findings support our study hypothesis.
Short sleepers had, on average, higher BMI and hypertension compared to normal sleepers. Several studies have suggested that short sleep duration is a risk factor for hypertension and metabolic diseases [33,34,35,36,37,38,39]. For instance, Gangwisch et al. [34] reported a positive association between short sleep duration and hypertension in adults, even after adjusting for BMI and diabetes history. A study by Wang et al. indicated that both short and long sleep durations are associated with higher risks of hypertension [35]. For patients with insomnia, those with objectively short sleep duration are particularly susceptible to developing hypertension [36] and cardiovascular disease, possibly due to their state of hyperarousal, indicated by elevated hypothalamic–pituitary–adrenal axis (HPA) and sympathetic activity [7]. Short sleep duration is also associated with the occurrence of overweight and obesity, with sleep duration of less than seven hours linked to a nearly two-fold increased rate of overweight and obesity compared to a 7–9 h sleep duration [38]. Furthermore, studies have shown that individuals with shorter sleep duration tend to have higher BMI [40].
Diet, particularly the intake of micronutrients, such as amino acids, is a crucial factor influencing sleep quality and can contribute to insomnia [41]. In the present study, although the protein percentage intake was the same between groups, short sleepers consumed relatively lower levels of the aromatic amino acids (AAAs) phenylalanine and tyrosine compared to the normal group. These findings are in line with a recent study which showed a significantly positive association between sleep duration and the intake of AAAs in adults with normal BMI [42]. Furthermore, significantly less valine, a branch-chain amino acid (BCAA), was consumed by the participants with a short sleep duration. BCAAs are involved in the de novo synthesis of glutamate and GABA, two neurotransmitters known to influence sleep [43,44]. Indeed, there is some evidence in patients with insomnia suggesting a correlation between reduced GABA levels and shorter sleep duration [45].
To investigate the role of microbiota-derived metabolites in insomnia, this study examined metabolites from the “microbial metabolism in diverse environments” pathway, specifically benzophenone, pyrogallol, 5-aminopental, butyl acrylate, kojic acid, DCA, trans-anethole, and 5-carboxyvanillic acid. From these metabolites, only secondary bile acids, such as DCA, have been previously reported to be related to sleep. DCA, in the current analysis, was detected in higher levels in short sleepers. DCA is generated as a result of biotransformation (7α/β-dehydroxylation) of the primary bile acid, cholic acid, by specific bacteria in the intestinal microbiota, such as species belonging to the Clostridium genus (e.g., C. scindens, C. hiranonis, C. hylemonae and C. sordelli) [46]. Unlike rodents, the human liver is incapable of 7α-hydroxylating secondary bile acids returning to the liver via the portal vein, and, thus, secondary bile acids accumulate to high levels in the bile of some humans [47]. The current findings are in line with a recent study which integrated multi-omics data from two human cohorts and which found an association between insomnia and higher levels of secondary bile acids [48], suggesting that insomnia might have a significant impact on the gut microbiota–bile acid axis.
Higher levels of DCA, shown here in short sleepers, have been previously shown to cause oxidative stress in the intestinal epithelial cells, leading to increased secretion of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α, in colonocytes and causing damage to the mucosal layer of the colon [25]. Furthermore, mice fed with DCA developed inflammation in the colon, accompanied by dysbiosis of the intestinal microbiota [49]. Given that insomnia of short sleep duration is also positively associated with inflammation and dysbiosis, DCA could be considered a potential biomarker for this disease phenotype.
The strengths of the study included its comprehensive approach, combining various methods such as sleep assessment, dietary analysis, and advanced metabolomics, to gain a detailed understanding of the interplay between sleep patterns, diet and metabolism. The study was limited by the small sample size, which may impact the generalizability of the findings, the self-reported dietary data which could introduce bias, and the focus on older adults which limits generalization to younger age groups.
5. Conclusions
Overall, this study found that older adults with short sleep-duration insomnia had higher BMI and blood pressure compared with the normal sleep-duration group. Moreover, short sleepers consumed fewer amino acids and presented higher fecal levels of DCA, a secondary bile acid produced by microbiota fermentation. Further investigation will be needed to elucidate the mechanisms connecting sleep, diet, and microbiota metabolites and the role of DCA as a biomarker in insomnia.
Acknowledgments
We thank all the participants in this study.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/biom14040419/s1, Supplementary Data S1: Participants’ nutritional intake; Supplementary Data S2: Metabolites.
Author Contributions
Conceptualization, T.S., I.H., M.A. and S.T.; methodology, F.M. and S.T.; formal analysis, C.E.; investigation, C.E. and F.M.; resources, T.S., I.H., M.A. and S.T.; writing—original draft preparation, F.M. and C.E.; writing—review and editing, F.M.; supervision, F.M. and S.T.; funding acquisition, T.S., I.H., M.A. and S.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the institutional review board (IRB) of the Faculty of Social Welfare and Health Sciences at the University of Haifa (approval number 026/17 approval date 19.1.2017).
Informed Consent Statement
Informed consent was obtained from all participants involved in the study.
Data Availability Statement
Data are contained within the article and Supplementary Materials.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding Statement
This research was funded by the Israeli Ministry of Science and Technology, grant number 3-13607.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Shochat T., Ancoli-Israel S. Chapter 153—Insomnia in Older Adults. In: Kryger M., Roth T., Dement W.C., editors. Principles and Practice of Sleep Medicine. 6th ed. Elsevier; Amsterdam, The Netherlands: 2017. pp. 1503–1509.e4. [Google Scholar]
- 2.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. American Psychiatric Association Publishing; Washington, DC, USA: 2022. DSM-5-TR. [Google Scholar]
- 3.Karlson C.W., Gallagher M.W., Olson C.A., Hamilton N.A. Insomnia Symptoms and Well-Being: Longitudinal Follow-Up. Health Psychol. 2013;32:311–319. doi: 10.1037/a0028186. [DOI] [PubMed] [Google Scholar]
- 4.Zaslavsky O., LaCroix A.Z., Hale L., Tindle H., Shochat T. Longitudinal Changes in Insomnia Status and Incidence of Physical, Emotional, or Mixed Impairment in Postmenopausal Women Participating in the Women’s Health Initiative (WHI) Study. Sleep Med. 2015;16:364–371. doi: 10.1016/j.sleep.2014.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Patel D., Steinberg J., Patel P. Insomnia in the Elderly: A Review. J. Clin. Sleep Med. 2018;14:1017–1024. doi: 10.5664/jcsm.7172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalmbach D.A., Pillai V., Arnedt J.T., Drake C.L. DSM-5 Insomnia and Short Sleep: Comorbidity Landscape and Racial Disparities. Sleep. 2016;39:2101–2111. doi: 10.5665/sleep.6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vgontzas A.N., Fernandez-Mendoza J., Liao D., Bixler E.O. Insomnia with Objective Short Sleep Duration: The Most Biologically Severe Phenotype of the Disorder. Sleep Med. Rev. 2013;17:241–254. doi: 10.1016/j.smrv.2012.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fernandez-Mendoza J., Baker J.H., Vgontzas A.N., Gaines J., Liao D., Bixler E.O. Insomnia Symptoms with Objective Short Sleep Duration Are Associated with Systemic Inflammation in Adolescents. Brain Behav. Immun. 2017;61:110–116. doi: 10.1016/j.bbi.2016.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jarrin D.C., Ivers H., Lamy M., Chen I.Y., Harvey A.G., Morin C.M. Cardiovascular Autonomic Dysfunction in Insomnia Patients with Objective Short Sleep Duration. J. Sleep Res. 2018;27:e12663. doi: 10.1111/jsr.12663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bathgate C.J., Edinger J.D., Krystal A.D. Insomnia Patients With Objective Short Sleep Duration Have a Blunted Response to Cognitive Behavioral Therapy for Insomnia. Sleep. 2016;40:zsw012. doi: 10.1093/sleep/zsw012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Z., Wang Z., Lu T., Chen W., Yan W., Yuan K., Shi L., Liu X., Zhou X., Shi J., et al. The Microbiota-Gut-Brain Axis in Sleep Disorders. Sleep Med. Rev. 2022;65:101691. doi: 10.1016/j.smrv.2022.101691. [DOI] [PubMed] [Google Scholar]
- 12.Liu B., Lin W., Chen S., Xiang T., Yang Y., Yin Y., Xu G., Liu Z., Liu L., Pan J., et al. Gut Microbiota as a Subjective Measurement for Auxiliary Diagnosis of Insomnia Disorder. Front. Microbiol. 2019;10:461839. doi: 10.3389/fmicb.2019.01770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ning J., Huang S.-Y., Chen S.-D., Zhang Y.-R., Huang Y.-Y., Yu J.-T. Investigating Casual Associations Among Gut Microbiota, Metabolites, and Neurodegenerative Diseases: A Mendelian Randomization Study. J. Alzheimers Dis. 2022;87:211–222. doi: 10.3233/JAD-215411. [DOI] [PubMed] [Google Scholar]
- 14.Johnson K.V.-A., Foster K.R. Why Does the Microbiome Affect Behaviour? Nat. Rev. Microbiol. 2018;16:647–655. doi: 10.1038/s41579-018-0014-3. [DOI] [PubMed] [Google Scholar]
- 15.Niazi M.K., Hassan F., Tufail T., Ismail M.A., Riaz K. The Role of Microbiome in Psychiatric Diseases (Insomnia and Anxiety/Depression) with Microbiological Mechanisms. Adv. Gut Microbiome Res. 2023;2023:e1566684. doi: 10.1155/2023/1566684. [DOI] [Google Scholar]
- 16.Ojeda J., Ávila A., Vidal P.M. Gut Microbiota Interaction with the Central Nervous System throughout Life. J. Clin. Med. 2021;10:1299. doi: 10.3390/jcm10061299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Godos J., Currenti W., Angelino D., Mena P., Castellano S., Caraci F., Galvano F., Del Rio D., Ferri R., Grosso G. Diet and Mental Health: Review of the Recent Updates on Molecular Mechanisms. Antioxidants. 2020;9:346. doi: 10.3390/antiox9040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou J., Wu X., Li Z., Zou Z., Dou S., Li G., Yan F., Chen B., Li Y. Alterations in Gut Microbiota Are Correlated With Serum Metabolites in Patients With Insomnia Disorder. Front. Cell. Infect. Microbiol. 2022;12:722662. doi: 10.3389/fcimb.2022.722662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Humer E., Pieh C., Brandmayr G. Metabolomics in Sleep, Insomnia and Sleep Apnea. IJMS. 2020;21:7244. doi: 10.3390/ijms21197244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Haimov I., Magzal F., Tamir S., Lalzar M., Asraf K., Milman U., Agmon M., Shochat T. Variation in Gut Microbiota Composition Is Associated with Sleep Quality and Cognitive Performance in Older Adults with Insomnia. Nat. Sci. Sleep. 2022;14:1753–1767. doi: 10.2147/NSS.S377114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jariyasopit N., Khoomrung S. Mass Spectrometry-Based Analysis of Gut Microbial Metabolites of Aromatic Amino Acids. Comput. Struct. Biotechnol. J. 2023;21:4777–4789. doi: 10.1016/j.csbj.2023.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y., Hou Y., Wang G., Zheng X., Hao H. Gut Microbial Metabolites of Aromatic Amino Acids as Signals in Host–Microbe Interplay. Trends Endocrinol. Metab. 2020;31:818–834. doi: 10.1016/j.tem.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 23.Guzior D.V., Quinn R.A. Review: Microbial Transformations of Human Bile Acids. Microbiome. 2021;9:140. doi: 10.1186/s40168-021-01101-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bernstein C., Holubec H., Bhattacharyya A.K., Nguyen H., Payne C.M., Zaitlin B., Bernstein H. Carcinogenicity of Deoxycholate, a Secondary Bile Acid. Arch. Toxicol. 2011;85:863–871. doi: 10.1007/s00204-011-0648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu L., Dong W., Wang S., Zhang Y., Liu T., Xie R., Wang B., Cao H. Deoxycholic Acid Disrupts the Intestinal Mucosal Barrier and Promotes Intestinal Tumorigenesis. Food Funct. 2018;9:5588–5597. doi: 10.1039/C8FO01143E. [DOI] [PubMed] [Google Scholar]
- 26.Magzal F., Even C., Haimov I., Agmon M., Asraf K., Shochat T., Tamir S. Associations between Fecal Short-Chain Fatty Acids and Sleep Continuity in Older Adults with Insomnia Symptoms. Sci. Rep. 2021;11:4052. doi: 10.1038/s41598-021-83389-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kukull W.A., Larson E.B., Teri L., Bowen J., McCormick W., Pfanschmidt M.L. The Mini-Mental State Examination Score and the Clinical Diagnosis of Dementia. J. Clin. Epidemiol. 1994;47:1061–1067. doi: 10.1016/0895-4356(94)90122-8. [DOI] [PubMed] [Google Scholar]
- 28.Shahar D., Fraser D., Shai I., Vardi H. Development of a Food Frequency Questionnaire (FFQ) for an Elderly Population Based on a Population Survey. J. Nutr. 2003;133:3625–3629. doi: 10.1093/jn/133.11.3625. [DOI] [PubMed] [Google Scholar]
- 29.Cesbron N., Royer A.-L., Guitton Y., Sydor A., Le Bizec B., Dervilly-Pinel G. Optimization of Fecal Sample Preparation for Untargeted LC-HRMS Based Metabolomics. Metabolomics. 2017;13:99. doi: 10.1007/s11306-017-1233-8. [DOI] [Google Scholar]
- 30.Pence H.E., Williams A. ChemSpider: An Online Chemical Information Resource. J. Chem. Educ. 2010;87:1123–1124. doi: 10.1021/ed100697w. [DOI] [Google Scholar]
- 31.mzCloud—Advanced Mass Spectral Database. [(accessed on 11 January 2023)]. Available online: https://www.mzcloud.org/
- 32.Kanehisa M., Goto S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bock J.M., Vungarala S., Covassin N., Somers V.K. Sleep Duration and Hypertension: Epidemiological Evidence and Underlying Mechanisms. Am. J. Hypertens. 2021;35:3–11. doi: 10.1093/ajh/hpab146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gangwisch J.E., Heymsfield S.B., Boden-Albala B., Buijs R.M., Kreier F., Pickering T.G., Rundle A.G., Zammit G.K., Malaspina D. Short Sleep Duration as a Risk Factor for Hypertension. Hypertension. 2006;47:833–839. doi: 10.1161/01.HYP.0000217362.34748.e0. [DOI] [PubMed] [Google Scholar]
- 35.Wang Q., Xi B., Liu M., Zhang Y., Fu M. Short Sleep Duration Is Associated with Hypertension Risk among Adults: A Systematic Review and Meta-Analysis. Hypertens. Res. 2012;35:1012–1018. doi: 10.1038/hr.2012.91. [DOI] [PubMed] [Google Scholar]
- 36.Carter J.R., Grimaldi D., Fonkoue I.T., Medalie L., Mokhlesi B., Cauter E.V. Assessment of Sympathetic Neural Activity in Chronic Insomnia: Evidence for Elevated Cardiovascular Risk. Sleep. 2018;41:zsy048. doi: 10.1093/sleep/zsy048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Zhang B., Zhou Y., Wang D., Liu X., Li L., Wang T., Zhang Y., Jiang M., Tang H., et al. Gut Microbiota Changes and Their Relationship with Inflammation in Patients with Acute and Chronic Insomnia. Nat. Sci. Sleep. 2020;12:895–905. doi: 10.2147/NSS.S271927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li Q. The Association between Sleep Duration and Excess Body Weight of the American Adult Population: A Cross-Sectional Study of the National Health and Nutrition Examination Survey 2015–2016. BMC Public Health. 2021;21:335. doi: 10.1186/s12889-021-10369-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Javaheri S., Storfer-Isser A., Rosen C.L., Redline S. The Association of Short and Long Sleep Durations with Insulin Sensitivity In Adolescents. J. Pediatr. 2011;158:617. doi: 10.1016/j.jpeds.2010.09.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hur S., Oh B., Kim H., Kwon O. Associations of Diet Quality and Sleep Quality with Obesity. Nutrients. 2021;13:3181. doi: 10.3390/nu13093181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rostami H., Khayyatzadeh S.S., Tavakoli H., Bagherniya M., Mirmousavi S.J., Farahmand S.K., Tayefi M., Ferns G.A., Ghayour-Mobarhan M. The Relationship between Adherence to a Dietary Approach to Stop Hypertension (DASH) Dietary Pattern and Insomnia. BMC Psychiatry. 2019;19:234. doi: 10.1186/s12888-019-2220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Noori S., Nadery M., Ghaffarian-Ensaf R., Khadem A., Mirzaei K., Keshavarz S.A., Movahedi A. The Relationship between the Intake of Branched-Chain and Aromatic Amino Acids and Individuals’ Sleep Quality Based on Body Mass Index, Gender, and Age. J. Health Popul. Nutr. 2023;42:47. doi: 10.1186/s41043-023-00383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Falup-Pecurariu C., Diaconu Ș., Țînț D., Falup-Pecurariu O. Neurobiology of Sleep (Review) Exp. Ther. Med. 2021;21:272. doi: 10.3892/etm.2021.9703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Holeček M. Branched-Chain Amino Acids in Health and Disease: Metabolism, Alterations in Blood Plasma, and as Supplements. Nutr. Metab. 2018;15:33. doi: 10.1186/s12986-018-0271-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Spiegelhalder K., Regen W., Nissen C., Feige B., Baglioni C., Riemann D., Hennig J., Lange T. Magnetic Resonance Spectroscopy in Patients with Insomnia: A Repeated Measurement Study. PLoS ONE. 2016;11:e0156771. doi: 10.1371/journal.pone.0156771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ridlon J.M., Kang D.J., Hylemon P.B., Bajaj J.S. Bile Acids and the Gut Microbiome. Curr. Opin. Gastroenterol. 2014;30:332–338. doi: 10.1097/MOG.0000000000000057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ridlon J.M., Kang D.-J., Hylemon P.B. Bile Salt Biotransformations by Human Intestinal Bacteria. J. Lipid Res. 2006;47:241–259. doi: 10.1194/jlr.R500013-JLR200. [DOI] [PubMed] [Google Scholar]
- 48.Jiang Z., Zhuo L., He Y., Fu Y., Shen L., Xu F., Gou W., Miao Z., Shuai M., Liang Y., et al. The Gut Microbiota-Bile Acid Axis Links the Positive Association between Chronic Insomnia and Cardiometabolic Diseases. Nat. Commun. 2022;13:3002. doi: 10.1038/s41467-022-30712-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu M., Cen M., Shen Y., Zhu Y., Cheng F., Tang L., Hu W., Dai N. Deoxycholic Acid-Induced Gut Dysbiosis Disrupts Bile Acid Enterohepatic Circulation and Promotes Intestinal Inflammation. Dig. Dis. Sci. 2021;66:568–576. doi: 10.1007/s10620-020-06208-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Materials.