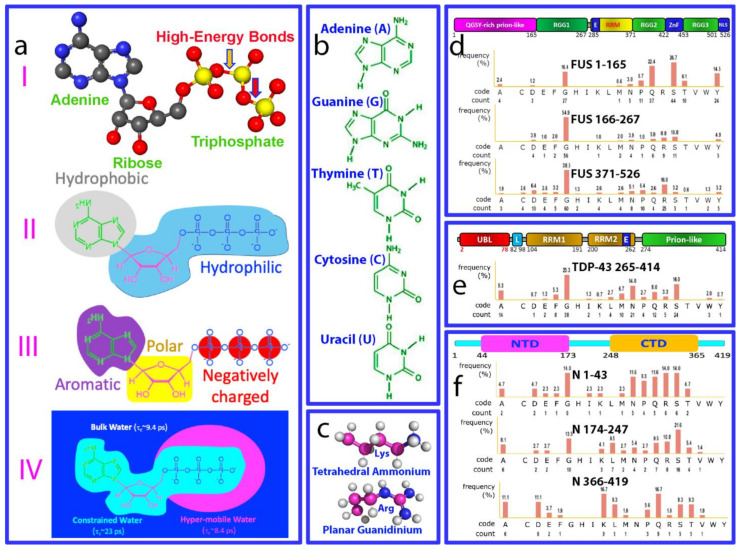
Figure 1.
Key properties of ATP and nucleic acids for modulating LLPS of FUS, TDP-43, and SARS-CoV-2 nucleocapsid proteins. (a) ATP has unique structural properties and thus may act as (I) energy currency by hydrolysis of high-energy bonds; (II) a biological hydrotrope with the presence of hydrophobic adenine and hydrophilic ribose and triphosphate; (III) a bivalent binder via the aromatic purine ring and highly negatively charged triphosphate chain; and (IV) a hydration mediator resulting from its unique hydration structure previously derived from the results of microwave dielectric spectroscopy, modeled to contain “constrained water” with a dielectric relaxation time (τc) of ~23 ps, as well as “hyper-mobile water” with a τc of ~8.4 ps, even smaller than that of bulk water (9.4 ps). Chemical structures of five nitrogenous bases in DNA and RNA (b), and of Arg and Lys side chains (c). Amino acid compositions of intrinsically disordered regions (IDRs) of FUS (d), TDP-43 (e), and SARS-CoV-2 nucleocapsid (N) proteins (f).