Abstract
Background:
To effectively counsel patients prior to shoulder arthroplasty, surgeons should understand the overall life trajectory and life expectancy of patients in the context of the patient’s shoulder pathology and medical comorbidities. Such an understanding can influence both operative and nonoperative decision-making and implant choices. This study evaluated 5-year mortality following shoulder arthroplasty in patients ≥65 years old and identified associated risk factors.
Methods:
We utilized Centers for Medicare & Medicaid Services Fee-for-Service inpatient and outpatient claims data to investigate the 5-year mortality rate following shoulder arthroplasty procedures performed from 2014 to 2016. The impact of patient demographics, including fracture diagnosis, year fixed effects, and state fixed effects; patient comorbidities; and hospital-level characteristics on 5-year mortality rates were assessed with use of a Cox proportional hazards regression model. A p value of <0.05 was considered significant.
Results:
A total of 108,667 shoulder arthroplasty cases (96,104 nonfracture and 12,563 fracture) were examined. The cohort was 62.7% female and 5.8% non-White and had a mean age at surgery of 74.3 years. The mean 5-year mortality rate was 16.6% across all shoulder arthroplasty cases, 14.9% for nonfracture cases, and 29.9% for fracture cases. The trend toward higher mortality in the fracture group compared with the nonfracture group was sustained throughout the 5-year postoperative period, with a fracture diagnosis being associated with a hazard ratio of 1.63 for mortality (p < 0.001). Medical comorbidities were associated with an increased risk of mortality, with liver disease bearing the highest hazard ratio (3.07; p < 0.001), followed by chronic kidney disease (2.59; p < 0.001), chronic obstructive pulmonary disease (1.92; p < 0.001), and congestive heart failure (1.90; p < 0.001).
Conclusions:
The mean 5-year mortality following shoulder arthroplasty was 16.6%. Patients with a fracture diagnosis had a significantly higher 5-year mortality risk (29.9%) than those with a nonfracture diagnosis (14.9%). Medical comorbidities had the greatest impact on mortality risk, with chronic liver and kidney disease being the most noteworthy. This novel longer-term data can help with patient education and risk stratification prior to undergoing shoulder replacement.
Level of Evidence:
Therapeutic Level III. See Instructions for Authors for a complete description of levels of evidence.
The utilization of total shoulder arthroplasty (TSA) has substantially increased in recent years1, with recent estimates suggesting a growth rate of approximately 5% to 9% per year2. This trend will likely continue, given increasing life expectancies and the exponentially increasing incidence rates of shoulder arthroplasty1,3.
Previous studies have reported complication rates following shoulder arthroplasty4,5; however, information is limited regarding long-term survival and patient outcomes6,7. Furthermore, the long-term value to the patient following shoulder arthroplasty is poorly defined. The first steps in understanding long-term value are simply to define patient survivorship following a shoulder replacement and to identify the risk factors associated with mortality. Understanding the differences in outcomes and survival rates between patients undergoing elective arthroplasty for degenerative indications and those undergoing arthroplasty for fracture has implications for resource allocation and is valuable information for orthopaedic surgeons and patients8-10.
Osteoporotic fractures present a substantial social and economic burden11. Proximal humeral fractures, which are common fragility fractures, account for 4% to 10% of adult fracture cases12. Prior studies have established 1-year mortality rates of 7.83% to 13.05% following a proximal humeral fracture13,14, but longer-term mortality risk following operative treatment has not been reported. Understanding the population of patients who are living after having undergone a shoulder arthroplasty for fracture has several health policy and planning implications1,15. As patients live longer, the incidence of osteoporotic fractures will continue to increase11, and it will be important to better define longer-term outcomes for use in patient and family education.
Prior studies have evaluated in-hospital, 30-day, 90-day, and 1-year mortality following shoulder replacement7,10,16-18, but no prior work, to our knowledge, has quantified mid- and long-term mortality. The purposes of the present study were to evaluate 5-year patient mortality following shoulder arthroplasty in patients ≥65 years old and to stratify this population into fracture and nonfracture groups. To provide some context for these mortality rates, we compared the population of patients who underwent shoulder arthroplasty with the average Medicare beneficiary. We also compared the shoulder arthroplasty population with a total hip arthroplasty (THA) population, as THA is another orthopaedic procedure that is performed for both elective indications and fragility fracture diagnoses. Furthermore, we identified factors associated with an increased risk of mortality in the first 5 years after shoulder arthroplasty.
Materials and Methods
Data Source
We utilized 100% Centers for Medicare & Medicaid Services (CMS) Fee-for-Service (FFS) inpatient and outpatient claims data regarding shoulder arthroplasty procedures performed from 2014 through the third quarter (Q3) of 2016. We studied the 5-year mortality rate in this population with use of 100% CMS Medicare enrollment data from 2014 to Q3 of 2021, with 2021 Q3 data being the most recent data available at the time of our analysis.
We included primary shoulder arthroplasty cases that were coded as inpatient procedures using Diagnosis-Related Group (DRG) codes 483 and 484 and those that were coded as outpatient procedures using Current Procedural Terminology (CPT) code 23472. Outpatient TSAs made up a small fraction of total cases because CMS removed TSA from the inpatient-only list in 2021. The primary International Classification of Diseases, 9th and 10th Revision, Procedure Coding System (ICD-9/10-PCS) procedure code for each case was utilized to verify the use of shoulder arthroplasty. We further divided the TSAs into fracture and nonfracture cases on the basis of the primary International Classification of Diseases, 9th and 10th Revision, Clinical Modification (ICD-9/10-CM) diagnosis code for each case. Because anatomic shoulder arthroplasty and reverse shoulder arthroplasty utilize the same CPT code, we could not develop a consistent methodology to reliably distinguish by arthroplasty type.
Medicare enrollees include people with disabilities and people with end-stage renal disease in addition to seniors. We therefore excluded Medicare patients <65 years old in order to evaluate the impact of age on mortality rates without introducing bias from the skewed distribution of comorbidities among patients <65 years old in the database. For each case, we captured the comorbidity status of the patient at the time of the procedure with use of the complete list of ICD-9/10-CM codes billed with the arthroplasty claim.
To better understand mortality rates following shoulder arthroplasty, we utilized data from the aforementioned Medicare claims and enrollment database to calculate the 5-year mortality rate following THAs performed for fracture as well as that following elective THAs. THAs were identified through DRG 469 and 470, CPT 27130, and primary ICD-9/10-PCS codes, and fracture cases were identified through primary ICD-9/10-CM codes. Additionally, we evaluated mortality rates for all Medicare beneficiaries during the study period.
Statistical Analysis
A Cox proportional hazards regression model was utilized to evaluate the drivers of mortality in the shoulder arthroplasty cohort. The time to death after the index arthroplasty was the dependent variable. We controlled for case-level demographic and comorbidity characteristics and hospital-level characteristics as independent variables and quantified their impact on mortality. Specifically, case-level demographics comprised age, sex, White versus non-White race (as reported in the database), fracture versus nonfracture, year fixed effects, and state fixed effects; case-level comorbidities comprised a body mass index (BMI) of >40 kg/m2, congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), cancer, diabetes, kidney disease, and liver disease; and hospital-level characteristics comprised teaching status, urban status, and bed size. We examined the proportional hazards assumption for indicators of fracture and mortality risk over time (Fig. 1). Significance was set at p < 0.05.
Fig. 1.
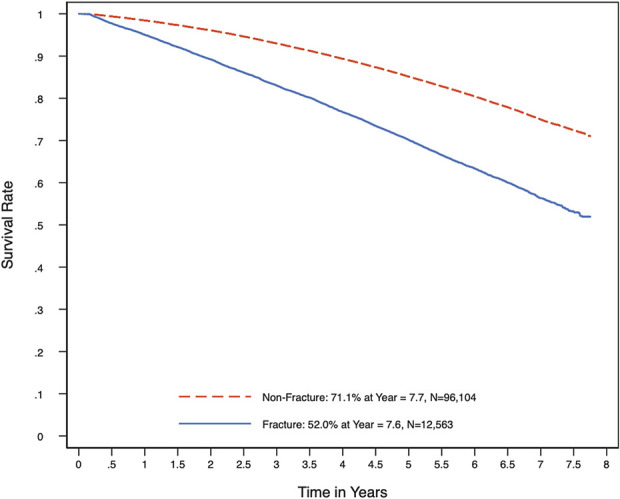
Survival rates among patients who underwent shoulder arthroplasty for fracture versus nonfracture.
Results
The study included 108,667 shoulder arthroplasty cases, of which 96,104 were nonfracture cases and 12,563 were fracture cases. The mortality rate was 16.6% in the shoulder arthroplasty cohort overall, 29.9% in the fracture group, and 14.9% in the nonfracture group. Table I presents full demographic data for the study cohort.
TABLE I.
Demographic and Comorbidity Profile of the Shoulder Arthroplasty Cohort*
| Variable | All TSAs | Nonfracture | Fracture | P Value |
|---|---|---|---|---|
| Case-level demographics | ||||
| Volume (no. [%]) | 108,667 (100) | 96,104 (88) | 12,563 (12) | NA |
| 5-year mortality (%) | 16.6 | 14.9 | 29.9 | <0.001 |
| Age at surgery, all† (yr) | 74.3 | 74.0 | 76.6 | <0.001 |
| Age at death, if deceased† (yr) | 81.0 | 80.5 | 82.7 | <0.001 |
| Female (%) | 62.7 | 59.8 | 84.9 | <0.001 |
| Non-White (%) | 5.8 | 5.9 | 4.5 | <0.001 |
| Case-level comorbidities (%) | ||||
| BMI > 40 kg/m2 | 4.3 | 4.3 | 4.6 | 0.07 |
| CHF | 6.0 | 5.6 | 8.7 | <0.001 |
| COPD | 10.8 | 10.6 | 12.4 | <0.001 |
| Cancer | 1.4 | 1.2 | 2.3 | <0.001 |
| Diabetes | 21.7 | 21.1 | 26.6 | <0.001 |
| Kidney disease | 1.4 | 1.0 | 3.8 | <0.001 |
| Liver disease | 0.4 | 0.4 | 0.7 | <0.001 |
| Hospital-level characteristics | ||||
| Annual surgical volume† (no.) | 2,622 | 2,506 | 2,167 | NA |
| Urban (%) | 79.9 | 80.4 | 82.9 | 0.03 |
| Bed size† (no.) | 215.4 | 218.1 | 237.2 | 0.002 |
| Teaching (%) | 37.4 | 37.5 | 40.9 | 0.01 |
NA = not applicable, BMI = body mass index, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease.
Values are given as the mean.
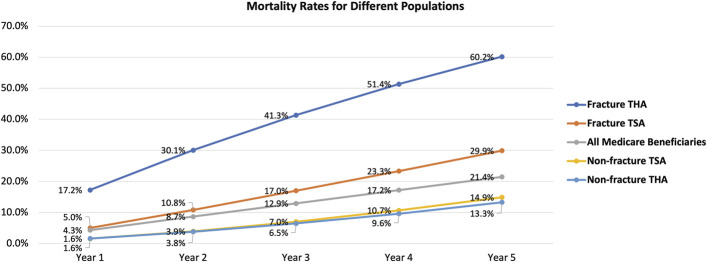
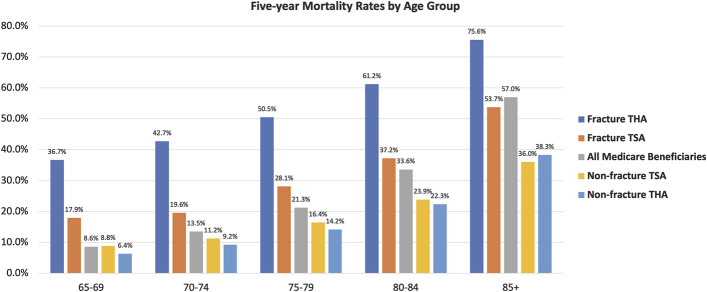
Patients with THA for fracture had the highest 5-year mortality rate at 60.2% (Fig. 2). Patients with shoulder arthroplasty for a fracture diagnosis had the second highest 5-year mortality rate at 29.9%. Patients with TSA and THA for a nonfracture diagnosis had the lowest 5-year mortality rates at 14.9% and 13.3%, respectively. The Medicare beneficiary mortality rate was 21.4%. Five-year mortality was more likely with increasing age; this trend was observed across all study populations (Fig. 3).
Fig. 2.
Five-year mortality rates, stratified by year, for each of the following populations: total shoulder arthroplasty (TSA) cases with a fracture diagnosis, TSA cases with a nonfracture diagnosis, total hip arthroplasty (THA) cases with a fracture diagnosis, THA cases with a nonfracture diagnosis, and all Medicare beneficiaries.
Fig. 3.
Five-year mortality rates, stratified by age category, for each of the following populations: total shoulder arthroplasty (TSA) cases with a fracture diagnosis, TSA cases with a nonfracture diagnosis, total hip arthroplasty (THA) cases with a fracture diagnosis, THA cases with a nonfracture diagnosis, and all Medicare beneficiaries.
In the Cox proportional hazards regression model, fracture cases were associated with an increased risk of 5-year mortality, as indicated by a hazard ratio of 1.63 (95% confidence interval [CI], 1.58 to 1.69; p < 0.001). Older age and a diagnosis of CHF, COPD, cancer, diabetes mellitus, kidney disease, or liver disease at the time of the index arthroplasty were each associated with an increased risk of mortality (Table II). Among the aforementioned comorbidities, liver disease was associated with the most increased risk of 5-year mortality, with an odds ratio of 3.07 (95% CI, 2.68 to 3.52; p < 0.001), followed by kidney disease, with an odds ratio of 2.59 (95% CI, 2.40 to 2.79; p < 0.001); COPD, with an odds ratio of 1.92 (95% CI, 1.85 to 1.99; p < 0.001); and CHF, with an odds ratio of 1.90 (95% CI, 1.82 to 1.99; p < 0.001). Elevated BMI was associated with a slightly increased mortality risk, with an odds ratio of 1.13 (95% CI, 1.06 to 1.21; p < 0.001). Female sex was associated with a decreased mortality risk, with an odds ratio of 0.78 (95% CI, 0.76 to 0.80; p < 0.001).
TABLE II.
Cox Proportional Hazards Regression Analysis of Factors Associated with Increased 5-Year Mortality Risk*
| Variable | Odds Ratio | 95% CI | P Value |
|---|---|---|---|
| Age | 1.09 | 1.08-1.09 | <0.001 |
| Female | 0.78 | 0.76-0.80 | <0.001 |
| Non-White | 0.94 | 0.88-0.99 | 0.03 |
| Fracture | 1.63 | 1.58-1.69 | <0.001 |
| BMI > 40 kg/m2 | 1.13 | 1.06-1.21 | <0.001 |
| CHF | 1.90 | 1.82-1.99 | <0.001 |
| COPD | 1.92 | 1.85-1.99 | <0.001 |
| Cancer | 1.84 | 1.69-2.01 | <0.001 |
| Diabetes | 1.35 | 1.31-1.39 | <0.001 |
| Kidney disease | 2.59 | 2.40-2.79 | <0.001 |
| Liver disease | 3.07 | 2.68-3.52 | <0.001 |
| Urban hospital | 0.94 | 0.89-0.99 | 0.01 |
| Hospital bed size | 1.00 | 1.00-1.00 | 0.24 |
| Teaching hospital | 1.00 | 0.97-1.04 | 0.90 |
CI = confidence interval, BMI = body mass index, CHF = congestive heart failure, COPD = chronic obstructive pulmonary disease. In addition to the listed variables, this analysis controlled for year fixed effects and state fixed effects. A p value of <0.05 was significant.
Discussion
Our study demonstrated that patients ≥65 years old who underwent shoulder arthroplasty for fracture had significantly higher mortality rates than those who underwent shoulder arthroplasty for a nonfracture diagnosis. The trend toward higher mortality in the fracture group was sustained throughout the 5-year postoperative period (Fig. 1), with a fracture diagnosis being associated with 1.63 times higher odds of mortality (p < 0.001; Table II). Older age and medical comorbidities were the 2 other main risk factors for 5-year mortality. Although this study did not comprehensively explore the long-term value of shoulder arthroplasty, as we were unable to evaluate patient-reported outcomes, we believe that this analysis is a first step, providing novel data that can help surgeons to frame preoperative surgical discussions that include overall mortality risk and potential patient-specific risk factors associated with mortality.
Our findings suggest that increased 5-year mortality risk following shoulder arthroplasty is multifactorial. Fragility fracture and medical comorbidities both contributed to increased mortality risk. Although it was out of the scope of this study to evaluate mortality risk in patients with nonoperatively treated fractures, proximal humeral fragility fractures that do not require surgery or hospital admission are still substantial injuries with potential sequelae beyond diminished shoulder function13. As a result of the limitations of the Medicare database, we did not have the ability to reliably isolate proximal humeral fractures that were treated nonoperatively or with open reduction and internal fixation (ORIF), and therefore such cases were not included in the analysis. Furthermore, given the retrospective nature of this analysis, we felt that there would have been inherit bias introduced with regard to patient age and comorbidity status when evaluating and comparing proximal humeral fracture cases treated nonoperatively versus those treated with ORIF.
We identified a 5-year mortality risk for patients undergoing shoulder arthroplasty for fracture (29.9%) that was lower than that for patients undergoing THA for fracture (60.2%). However, these rates were higher than the rate for the average Medicare beneficiary (21.4%), indicating that fragility fractures are associated with an increased patient mortality risk at 5 years even when treated operatively (Fig. 2). This increased mortality risk in the fragility fracture population is consistent with previous literature14,18-20. Brown et al.19 reported that a fragility fracture at any site is associated with an increased 1-year mortality risk and a diminished 6-year survival probability. Furthermore, consistent with the findings of the present study, Brown et al. reported that a hip fragility fracture is associated with a higher mortality risk than a non-hip fragility fracture21.
In the present study, we found lower rates of 5-year mortality among patients who underwent elective shoulder and hip arthroplasty for a nonfracture diagnosis (14.9% and 13.3%, respectively; Fig. 2) than among other patient populations. Short-term mortality risk following primary shoulder arthroplasty has been well defined in the literature, with rates of 0.09% for in-hospital mortality16, 0.25% for 30-day mortality17, and 0.8% to 1.3% for 90-day mortality18,22. Longer-term mortality risk following TSA has not been directly studied, but indirect data can be gathered from prior literature. Sheth et al.23 evaluated 10-year clinical outcomes following reverse shoulder arthroplasty. Of their initial cohort of 471 patients, they found that 225 had died prior to follow-up, indicating a 47.8% 10-year mortality rate in this population. Evans et al.6 evaluated the 20-year outcomes of 1 implant design following anatomic shoulder arthroplasty. Of the 40 patients in their cohort, 29 had died, indicating a 72.5% mortality rate at the 20-year follow-up. One downside of both of these studies is that the low numbers of patients can introduce some degree of statistical fragility to the findings regarding mortality. Our cohort of 96,104 nonfracture shoulder arthroplasty cases demonstrated a 14.9% mortality rate at 5 years.
Our finding that the highest risks of 5-year mortality were associated with medical comorbidities is consistent with that of previous studies. Singh et al. found that an underlying tumor diagnosis and a higher medical comorbidity index were predisposing factors for increased mortality risk following TSA22. Garcia et al.24 identified a 7.6% rate of malnutrition (albumin <3.5 g/dL) among patients undergoing shoulder arthroplasty; malnutrition was associated with postoperative blood transfusion, extended length of stay, and mortality. Griffin et al.25 showed that obesity in patients undergoing TSA was associated with higher costs, perioperative respiratory complications, and increased length of stay; however, there was no significant difference in postoperative mortality rates between patients with and without obesity. Comorbid cardiac disease has been found to be an independent predictor of mortality, whereas peripheral vascular disease has been associated with increased complication rates following TSA17. The present study also demonstrated that CHF was associated with 1.90 times higher odds of 5-year mortality (p < 0.001). However, these odds of 5-year mortality were far exceeded by those associated with liver disease (3.07; p < 0.001) and kidney disease (2.59; p < 0.001; Table II). We were unable to reliably subdivide these comorbidities into further entities. This manifested as broad CIs for the hazard ratio for some comorbidities, which was the most pronounced for liver disease. Therefore, it should be understood that these findings represent overall risks and that individual patient odds may vary depending on the nature and severity of the comorbid condition. Nonetheless, preoperative comorbidity management may have the potential to impact short and longer-term mortality risk following shoulder arthroplasty and should be taken into consideration during preoperative counseling.
Given that the present study was based solely on data from the Medicare database, we were unable to evaluate the effects of insurance status or patient socioeconomic background on mortality. Interestingly, Like et al.26 found that Medicaid insurance status was a risk factor for morbidity and mortality following total shoulder arthroplasty, but their analysis was based on short-term outcomes (i.e., 30 and 90-day readmission rates). Additional research regarding the role of socioeconomic factors in mortality and patient outcomes following shoulder arthroplasty is necessary.
Because of the limitations of the Medicare database, we were unable to isolate all factors that could affect 5-year mortality rates, such as socioeconomic status, other medical treatments, nonorthopaedic surgical procedures, and subsequent traumas. Along these lines, we were not able to accurately identify the cause of death for all patients and therefore did not include this information in our analysis. We were also unable to consistently and reproducibly stratify data by arthroplasty type because anatomic shoulder arthroplasty and reverse shoulder arthroplasty utilize the same CPT code. Given similar limitations in coding, we were unable to reliably distinguish between primary diagnoses of osteoarthritis and rotator cuff arthropathy. The comorbidity status of the patient at the time of procedure was captured with use of the complete list of ICD-9/10-CM codes billed with the arthroplasty claim; such data were subject to potential errors caused by inaccurate billing or comorbidity documentation. Given that the majority of arthroplasty procedures for proximal humeral fractures may have been performed in patients admitted to the hospital, a bias toward cases with more complete comorbidity documentation in the fracture population is possible. In general, comorbidities are underdocumented in the hospital setting, and therefore our study may have underrepresented the true magnitude of the role of medical comorbidities in mortality.
We were not able to include clinical outcomes or patient satisfaction in our analysis. Although determining mortality rates is an important start to understanding the long-term value of care, quality-adjusted life years are an important consideration that we were unable to evaluate in our analysis. Finally, we were unable to accurately isolate a population of patients who were treated nonoperatively for a fragility fracture of their shoulder or hip and therefore were unable to provide a nonoperative control group for fracture when considering patients with a fragility fracture.
Conclusions
There was an overall 16.6% risk of mortality at 5 years following shoulder arthroplasty, which was lower than the mean Medicare beneficiary 5-year mortality rate of 21.4%. Shoulder arthroplasty for fracture had a higher 5-year mortality risk at 29.9%. This, however, was not as substantial as the 60.2% 5-year mortality rate following hip arthroplasty for fracture. Our findings are consistent with prior work showing an increased mortality risk following a fragility fracture. Medical comorbidities had the greatest impact on mortality risk, with chronic liver and kidney disease being the most noteworthy. This study provides novel longer-term data to help with patient education and risk stratification prior to a shoulder replacement for fracture or other elective indications.
Acknowledgments
Note: Members of the Avant-garde Health and Codman Shoulder Society Value-Based Care Group include (in alphabetical order): Joseph A. Abboud, MD, Rothman Institute, Thomas Jefferson University Hospital, Philadelphia, PA; April D. Armstrong, MD, Department of Orthopaedics and Rehabilitation, Bone and Joint Institute, Penn State Milton S. Hershey Medical Center, Hershey, PA; Robert M. Belniak, MD, Department of Orthopaedic Surgery and Sports Medicine, Starling Physicians Group, New Britain, CT; Matthew J. Best, MD, Department of Orthopaedic Surgery, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, Baltimore, MD; John Costouros, MD, California Shoulder Institute, Menlo Park, CA; Ana Paula Beck da Silva Etges, PhD, Avant-garde Health, Boston, MA; Mohamad Fares, Rothman Institute, Thomas Jefferson University Hospital, Philadelphia, PA; Catherine J. Fedorka, MD, Cooper Bone and Joint Institute, Camden, NJ; Michael B. Gottschalk, MD, Department of Orthopaedic Surgery, Emory University, Atlanta, GA; Derek A. Haas, MBA, Avant-garde Health, Boston, MA; Porter Jones, MD, MBA, Avant-garde Health, Boston, MA; Adam Z. Khan, MD, Department of Orthopaedic Surgery, Southern California Permanente Medical Group, Panorama City, California; Jacob M. Kirsch, MD, Department of Orthopaedic Surgery, New England Baptist Hospital, Tufts University, Boston, MA; Harry Liu, PhD, Avant-garde Health, Boston, MA; Eric C. Makhni, MD, MBA, Department of Orthopaedic Surgery, Henry Ford Health System, Detroit, MI; Augustus Mazzocca, MD, Department of Orthopaedic Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Evan A. O’Donnell, MD, Boston Shoulder Institute, Department of Orthopaedic Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Jason E. Simon, MD, MBA, Department of Orthopaedic Surgery, Newton-Wellesley Hospital, Harvard Medical School, Boston, Massachusetts; Uma Srikumaran, MD, MBA, MPH, Department of Orthopaedic Surgery, Johns Hopkins Hospital, Johns Hopkins University School of Medicine, Baltimore, MD; Evan Stieler, MD, Boston Shoulder Institute, Department of Orthopaedic Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Gary F. Updegrove, MD, Bone and Joint Institute, Department of Orthopaedics and Rehabilitation, Penn State Milton S. Hershey Medical Center, Hershey, PA; Eric R. Wagner, MD, Department of Orthopaedic Surgery, Emory University, Atlanta, GA; Jon J.P. Warner, MD, Boston Shoulder Institute, Department of Orthopaedic Surgery, Massachusetts General Hospital, Harvard Medical School, Boston, MA; Jarret Woodmass, MD, FRCSC, Pan Am Clinic, Winnipeg, Manitoba, Canada; and Xiaoran Zhang, MA, Avant-garde Health, Boston, MA.
Footnotes
A list of the members of the Avant-garde Health and Codman Shoulder Society Value-Based Care Group is included in a note at the end of the article.
Disclosure: No external funding was received for this work. The Disclosure of Potential Conflicts of Interest forms are provided with the online version of the article (http://links.lww.com/JBJSOA/A622).
Contributor Information
Adam Z. Khan, Email: adamzkhan12@gmail.com.
Xiaoran Zhang, Email: luka@avantgardehealth.com.
Erlyn Macarayan, Email: ekmacarayan@gmail.com.
Matthew J. Best, Email: mbest8@jhmi.edu.
Catherine J. Fedorka, Email: cjfedorka@gmail.com.
Derek A. Haas, Email: derek@avantgardehealth.com.
April D. Armstrong, Email: aarmstrong@pennstatehealth.psu.edu.
Andrew Jawa, Email: andrewjawa@gmail.com.
Jason E. Simon, Email: jsimon2@partners.org.
Eric R. Wagner, Email: eric.r.wagner@emory.edu.
Momin Malik, Email: momin@avantgardehealth.com.
Michael B. Gottschalk, Email: Michael.gottschalk@emoryhealthcare.org.
Gary F. Updegrove, Email: gupdegrove@pennstatehealth.psu.edu.
Jon J.P. Warner, Email: jwarner@mgh.harvard.edu.
Joseph A. Abboud, Email: abboudj@gmail.com.
Collaborators: Joseph A. Abboud, April D. Armstrong, Robert M. Belniak, Matthew J. Best, John Costouros, Ana Paula Beck da Silva Etges, Mohamad Fares, Catherine J. Fedorka, Michael B. Gottschalk, Derek A. Haas, Porter Jones, Adam Z. Khan, Jacob M. Kirsch, Harry Liu, Eric C. Makhni, Augustus Mazzocca, Evan A. O’Donnell, Jason E. Simon, Uma Srikumaran, Evan Stieler, Gary F. Updegrove, Eric R. Wagner, Jon J.P. Warner, Jarret Woodmass, and Xiaoran Zhang
References
- 1.Farley KX, Wilson JM, Kumar A, Gottschalk MB, Daly C, Sanchez-Sotelo J, Wagner ER. Prevalence of Shoulder Arthroplasty in the United States and the Increasing Burden of Revision Shoulder Arthroplasty. JB JS Open Access. 2021. Jul 14;6(3):e20.00156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Steinhaus ME, Shim SS, Lamba N, Makhni EC, Kadiyala RK. Outpatient total shoulder arthroplasty: A cost-identification analysis. J Orthop. 2018. May 7;15(2):581-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day JS, Lau E, Ong KL, Williams GR, Ramsey ML, Kurtz SM. Prevalence and projections of total shoulder and elbow arthroplasty in the United States to 2015. J Shoulder Elbow Surg. 2010. Dec 1;19:1115-20. [DOI] [PubMed] [Google Scholar]
- 4.Ravi V, Murphy RJ, Moverley R, Derias M, Phadnis J. Outcome and complications following revision shoulder arthroplasty: a systematic review and meta-analysis. Bone Joint Open. 2021. Aug;2(8):618-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Craig RS, Lane JCE, Carr AJ, Furniss D, Collins GS, Rees JL. Serious adverse events and lifetime risk of reoperation after elective shoulder replacement: population based cohort study using hospital episode statistics for England. BMJ. 2019. Feb 20;364:l298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Evans JP, Batten T, Bird J, Thomas WJ, Kitson JB, Smith CD. Survival of the Aequalis total shoulder replacement at a minimum 20-year follow-up: a clinical and radiographic study. J Shoulder Elbow Surg. 2021. Oct;30(10):2355-60. [DOI] [PubMed] [Google Scholar]
- 7.Abdelmalik BM, Hao KA, Turnbull LM, Wright TW, Wright JO, Farmer KW, Pazik M, King JJ. Survivorship after reverse total shoulder arthroplasty and predictors of 1-year and overall mortality. J Shoulder Elbow Surg. 2023. Jan;32(1):e1-10. [DOI] [PubMed] [Google Scholar]
- 8.Speerin R, Slater H, Li L, Moore K, Chan M, Dreinhöfer K, Ebeling PR, Willcock S, Briggs AM. Moving from evidence to practice: Models of care for the prevention and management of musculoskeletal conditions. Best Pract Res Clin Rheumatol. 2014. Jun;28(3):479-515. [DOI] [PubMed] [Google Scholar]
- 9.Gregory T, Hansen U, Emery RJ, Augereau B, Amis AA. Developments in shoulder arthroplasty. Proc Inst Mech Eng H. 2007. Jan;221(1):87-96. [DOI] [PubMed] [Google Scholar]
- 10.Singh JA, Ramachandran R. Persisting Racial Disparities in Total Shoulder Arthroplasty Utilization and Outcomes. J Racial Ethn Health Disparities. 2015;2015:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Khan AZ, Rames RD, Miller AN. Clinical Management of Osteoporotic Fractures. Curr Osteoporos Rep. 2018. Jun;16(3):299-311. [DOI] [PubMed] [Google Scholar]
- 12.Passaretti D, Candela V, Sessa P, Gumina S. Epidemiology of proximal humeral fractures: a detailed survey of 711 patients in a metropolitan area. J Shoulder Elbow Surg. 2017. Dec;26(12):2117-24. [DOI] [PubMed] [Google Scholar]
- 13.Bell JE, et al. Mortality after proximal humerus fractures. Journal of Shoulder and Elbow Surgery. 2016. Jun 1;25(6):E174. [Google Scholar]
- 14.Bergdahl C, Wennergren D, Ekelund J, Möller M. Mortality after a proximal humeral fracture. Bone Joint J. 2020. Nov;102-B(11):1484-90. [DOI] [PubMed] [Google Scholar]
- 15.Malik AT, Bishop JY, Neviaser AS, Beals CT, Jain N, Khan SN. Shoulder Arthroplasty for a Fracture Is Not the Same as Shoulder Arthroplasty for Osteoarthritis: Implications for a Bundled Payment Model. J Am Acad Orthop Surg. 2019. Dec 15;27(24):927-32. [DOI] [PubMed] [Google Scholar]
- 16.McCormick F, Nwachukwu BU, Kiriakopoulos EBS, Schairer WW, Provencher MT, Levy J. In-hospital mortality risk for total shoulder arthroplasty: A comprehensive review of the Medicare database from 2005 to 2011. Int J Shoulder Surg. 2015. Oct-Dec;9(4):110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Waterman BR, Dunn JC, Bader J, Urrea L, Schoenfeld AJ, Belmont PJ, Jr. Thirty-day morbidity and mortality after elective total shoulder arthroplasty: patient-based and surgical risk factors. J Shoulder Elbow Surg. 2015. Jan;24(1):24-30. [DOI] [PubMed] [Google Scholar]
- 18.Farng E, Zingmond D, Krenek L, Soohoo NF. Factors predicting complication rates after primary shoulder arthroplasty. J Shoulder Elbow Surg. 2011. Jun;20(4):557-63. [DOI] [PubMed] [Google Scholar]
- 19.Brown JP, Adachi JD, Schemitsch E, Tarride JE, Brown V, Bell A, Reiner M, Oliveira T, Motsepe-Ditshego P, Burke N, Slatkovska L. Mortality in older adults following a fragility fracture: real-world retrospective matched-cohort study in Ontario. BMC Musculoskelet Disord. 2021. Jan 23;22(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Downey C, Kelly M, Quinlan JF. Changing trends in the mortality rate at 1-year post hip fracture - a systematic review. World J Orthop. 2019. Mar 18;10(3):166-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JP, Adachi JD, Schemitsch E, Tarride JE, Brown V, Bell A, Reiner M, Oliveira T, Motsepe-Ditshego P, Burke N, Slatkovska L. Mortality in older adults following a fragility fracture: real-world retrospective matched-cohort study in Ontario. BMC Musculoskelet Disord. 2021. Jan 23;22(1):105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh JA, Sperling JW, Cofield RH. Ninety day mortality and its predictors after primary shoulder arthroplasty: an analysis of 4,019 patients from 1976-2008. BMC Musculoskelet Disord. 2011. Oct 12;12:231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sheth MM, Heldt BL, Spell JH, Vidal EA, Laughlin MS, Morris BJ, Elkousy HA, Edwards TB. Patient satisfaction and clinical outcomes of reverse shoulder arthroplasty: a minimum of 10 years’ follow-up. J Shoulder Elbow Surg. 2022. Apr;31(4):875-83. [DOI] [PubMed] [Google Scholar]
- 24.Garcia GH, Fu MC, Dines DM, Craig EV, Gulotta LV. Malnutrition: a marker for increased complications, mortality, and length of stay after total shoulder arthroplasty. J Shoulder Elbow Surg. 2016. Feb;25(2):193-200. [DOI] [PubMed] [Google Scholar]
- 25.Griffin JW, Novicoff WM, Browne JA, Brockmeier SF. Morbid obesity in total shoulder arthroplasty: risk, outcomes, and cost analysis. J Shoulder Elbow Surg. 2014. Oct;23(10):1444-8. [DOI] [PubMed] [Google Scholar]
- 26.Like BJ, White RS, Tangel V, Sullivan KJ, Arroyo NS, Stambough JB, Turnbull ZA. Medicaid payer status is associated with increased mortality and morbidity after inpatient shoulder arthroplasty: a multistate analysis, 2007-2014. Reg Anesth Pain Med. 2019. Feb;44(2):182-90. [DOI] [PubMed] [Google Scholar]