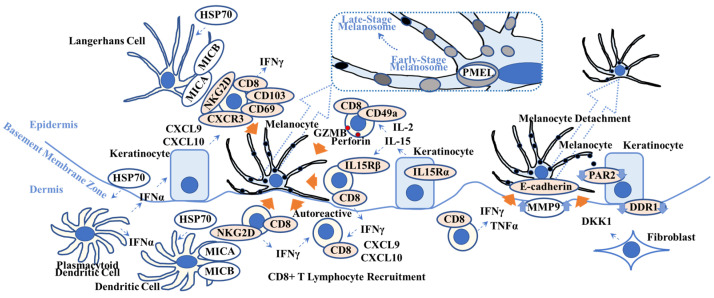
Figure 2.
Overview of possible vitiligo pathogenesis. This scheme shows key players in vitiligo pathogenesis. Heat-shock protein 70 (HSP70), one of the damage-associated molecular patterns, activates Langerhans cells and plasmacytoid dendritic cells that produce interferon-α (IFNα). IFNα stimulates keratinocytes to produce chemokine C-X-C motif ligand 10 (CXCL10) that activates CXCR3+ CD8+ T lymphocytes. IL-15 activates CD49a+ CD8+ T lymphocytes and induces granzyme B (GRMB) and perforin. The MHC class 1 polypeptide-related sequence A/B (MICA/MICB) on dendritic cells maintains natural killer cell receptor G2D (NKG2D)+ CD8+ T lymphocytes in the vitiligo lesion. IFNγ not only recruits CD8+ autoreactive T lymphocytes but also induces melanocyte detachment via E-cadherin disruption caused by an increase in matrix metalloproteinase 9 (MMP9). Discoidin domain receptor 1 (DDR1) forms a complex with E-cadherin, indicating its involvement in vitiligo pathogenesis. Dickkopf 1 (DKK1) decreases melanocyte function and proliferation and melanin uptake by keratinocytes via suppressed proteinase-activated receptor 2 (PAR2). Orange arrows indicate melanocyte apoptosis (all 5 left arrows), melanocyte detachment (second right arrow), and suppression of melanocyte function (far right arrow). (Dotted box) Pre-melanosome protein (PMEL) can be a target for autoreactive CD8+ T lymphocytes. Refer to Section 2.2. for details. TNFα = tumor necrosis factor-α; IL15R = interleukin-15 receptor; CD = cluster of differentiation.