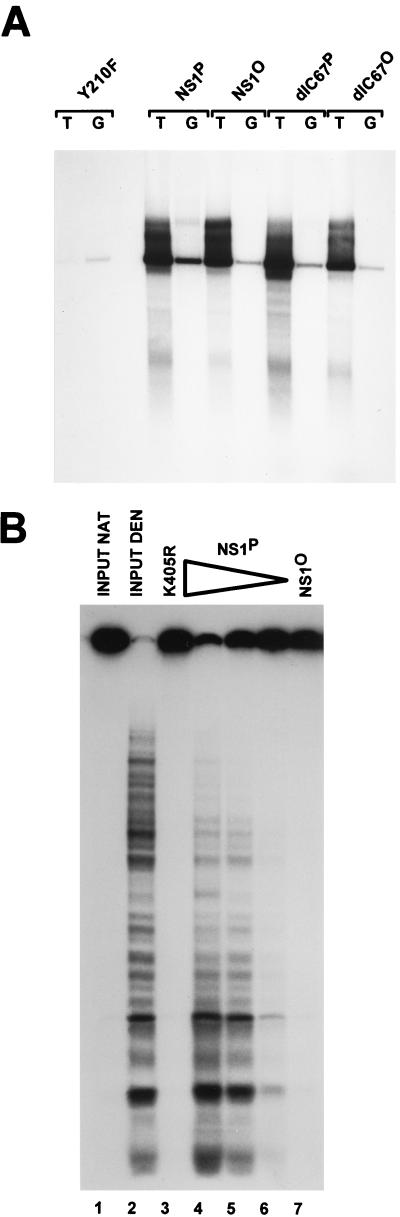
FIG. 1.
Comparison of replicative functions of native NS1P and dephosphorylated NS1O. NS1 was expressed from recombinant vaccinia viruses in HeLa-S3 cells and harvested at 18 h postinfection by preparing nuclear extracts. Dephosphorylated NS1O was obtained by treatment with calf intestine alkaline phosphatase. His-tagged native NS1P and phosphatase-treated NS1O were purified from nuclear extracts by Ni2+-NTA affinity chromatography. (A) NS1P and NS1O were compared for their capacities to support RCR in standard HeLa cell replication extracts, using plasmids containing the left-end active (T) or inactive (G) origin as substrates, in the presence of [32P]dATP. The reaction products were linearized with HindIII and analyzed by 0.8% agarose gel electrophoresis. The linkage tyrosine mutant Y210F (59) served as a negative control. The dlC67 mutant is replication competent (60) and was also analyzed in its native (P) and dephosphorylated (O) forms. (B) NS1P (100, 30, and 10 ng [lanes 4 to 6, respectively]) and NS1O (100 ng [lane 7]) were compared for their intrinsic helicase activities, using M13-VAR template, for 40 min at 37°C in the presence of 2 mM ATP. The ATP-binding site mutant K405R (57) served as a negative control. Reaction products were analyzed by native 7% PAGE in the presence of 0.1% SDS. Lanes 1 and 2, native (NAT) and denatured (DEN) input DNA, respectively.