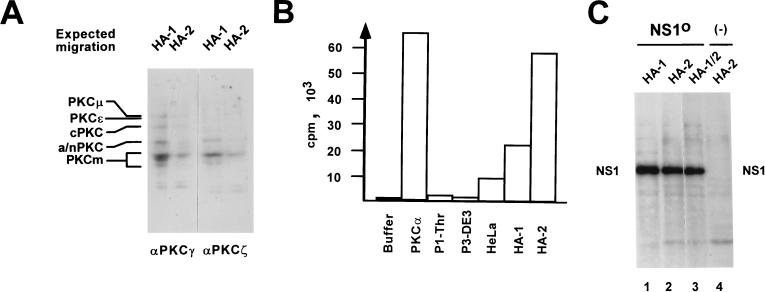
FIG. 7.
Detection of PKC within fractions HA-1 and HA-2 from HeLa cell extracts. (A) Western blot analysis of HA-1 or HA-2 after 10% SDS–PAGE, using peptide affinity-purified polyclonal antisera that were raised against the most-conserved regions of classical PKCs (PKCγ, amino acids 416 to 569; PKCζ, amino acids 310 to 444) and revealed the various PKC isoforms by cross-reaction. The expected migration positions of known PKC isoforms are given on the left: cPKC, classical PKCα, -β, and -γ (approximately 80 kDa); a/nPKC, atypical PKCι/λ and -ζ (70 to 72 kDa) and novel PKCδ, -η, and -θ (72 to 78 kDa). PKCɛ (90 kDa) and PKCμ (115 kDa), higher-molecular-mass novel PKCs; PKCm (approximately 45 kDa), PKC catalytic domains produced by proteolytic cleavage. (B) PKC activity assays (Amersham) of fractions used for reactivation of NS1O in replication reactions. Buffer, negative control in the absence of added protein components; PKCα, 10 ng of His tag-purified recombinant PKCα produced by recombinant vaccinia virus expression in HeLa cells; P1-Thr, fraction containing no NS1O-targeted protein kinase activity (see Fig. 2B); P3-DE3: fraction which proved able to phosphorylate but failed to reactivate NS1O (see Fig. 4); HeLa, standard HeLa cell replication extract; HA-1 and HA-2, NS1O-activating fractions in RCR assays (see Fig. 5). The values are expressed as transferred 32P-labeled substrate per 10 ng (recombinant PKCα) or 10 μg (HeLa cell fractions) of total effector proteins. (C) In vitro phosphorylation of 1 μg of NS1O with protein fractions HA-1 and HA-2, used individually or in combination in the presence of PKC cofactors. The reactions were performed in the presence of [γ-32P]ATP for 30 min at 37°C, and radiolabeled NS1 was revealed by autoradiography after SDS-PAGE. In lane 4, the NS1O substrate was omitted. The migration of NS1 is indicated.