Abstract
Glioblastoma multiforme (GBM) acquires resistance to bevacizumab (Bev) treatment. Bev affects angiogenic factors other than vascular endothelial growth factor (VEGF), which are poorly understood. We investigated changes in angiogenic factors under and after Bev therapy, including angiopoietin-1 (ANGPT1), angiopoietin-2 (ANGPT2), placental growth factor (PLGF), fibroblast growth factor 2, and ephrin A2 (EphA2). Fifty-four GBM tissues, including 28 specimens from 14 cases as paired specimens from the same patient obtained in three settings: initial tumor resection (naïve Bev), tumors resected following Bev therapy (effective Bev), and recurrent tumors after Bev therapy (refractory Bev). Immunohistochemistry assessed their expressions in tumor vessels and its correlation with recurrent MRI patterns. PLGF expression was higher in the effective Bev group than in the naïve Bev group (p = 0.024) and remained high in the refractory Bev group. ANGPT2 and EphA2 expressions were higher in the refractory Bev group than in the naïve Bev group (p = 0.047 and 0.028, respectively). PLGF expression was higher in the refractory Bev group compared with the naïve Bev group for paired specimens (p = 0.036). PLGF was more abundant in T2 diffuse/circumscribe patterns (p = 0.046). This is the first study to evaluate angiogenic factors other than VEGF during effective and refractory Bev therapy in patient-derived specimens.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10014-024-00481-0.
Keywords: Glioblastoma, Bevacizumab, Vascular endothelial growth factor, Alternative angiogenesis factor
Introduction
In Japan, bevacizumab (Bev), a monoclonal antibody against the potent angiogenic factor, vascular endothelial growth factor (VEGF) has been approved for newly diagnosed and recurrent high-grade gliomas. Bev has become one of the standard therapeutic modalities for glioblastoma multiforme (GBM), especially for recurrence, but overall survival (OS) is not prolonged in the clinical trials of phase 3 study [1, 2]. Unfortunately, the effect of Bev treatment does not last, regardless of drastic regression of tumor volume.
To explore the mechanism for Bev resistance, we have conducted comparative analyses of VEGF expression levels, tumor oxygenation, stemness, and immunoregulatory mechanisms using samples from patients that were naïve to, treated with, and refractory to Bev treatment using paired samples obtained from initial and recurrent surgery [3–6]. In these studies, the microvessel density in the tumor microenvironment (TME) under Bev treatment was significantly decreased with improvement in tumor oxygenation, and in the majority of Bev-refractory samples, tumor hypoxia was recovered with a paradoxical decrease in microvessel density [5]. Reactivation of VEGF may not, therefore, be initially involved in the acquisition of resistance to Bev and alternative and salvage angiogenesis pathways other than those involving VEGF can be induced in Bev therapy resistance [7]. Given that VEGF plays a pivotal role in tumor angiogenesis in GBM, other angiogenic factors, including fibroblast growth factor 2 (FGF2; also known as basic fibroblast growth factor, bFGF) [7], placental growth factor (PLGF) [8], platelet-derived growth factor (PDGF) [9], angiopoietin-1 (ANGPT1) [10–13], angiopoietin-2 (ANGPT2) [14–17], and Ephrin A2 (EphA2) [18–20] are also involved. However, very few reports have explored multiple angiogenic pathways using paired tumor tissues from the same patients with newly diagnosed and recurrent GBM, particularly surgical samples obtained during Bev treatment [8, 9, 20, 21].
To understand the mechanism of Bev resistance and to overcome its transient therapeutic efficacy, involvement of angiogenic factors other than VEGF need to be investigated during Bev therapy.
We addressed the following questions:
How are angiogenic factors other than VEGF involved during effective and refractory of Bev therapy?
Are salvage angiogenic pathways activated after Bev failure?
There are two common patterns of magnetic resonance imaging (MRI) in recurrent GBM after Bev therapy, T1-contrast enhancement and non-enhancement. Do enhancement/non-enhancement MR images represent involvement of salvage angiogenesis pathways after failure of Bev therapy?
Comparative analyses between initial and salvage surgery by analysis of surgical samples can help clarify the mechanism of action and resistance of Bev therapy. This study aimed to investigate the status and changes of non-VEGF angiogenic factors during Bev therapy by analysis of surgical samples from initial and salvage surgery. This is the first comparative analysis of alternative angiogenesis pathways using human surgical specimens derived from patients during effective and resistance stages of Bev therapy.
Patients and methods
Patient eligibility and sample collection
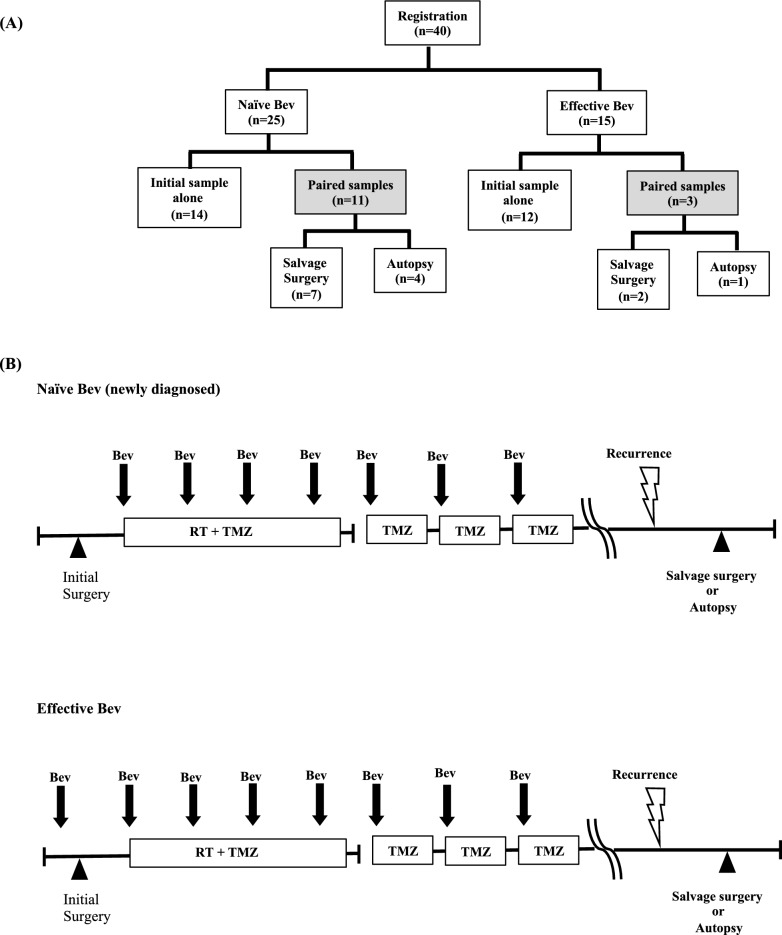
The present study used 54 GBM tissues from 40 patients obtained from three different settings as previously described [4]; 15 tumors were removed after neoadjuvant Bev administration, i.e., during Bev response (effective Bev), 25 were newly diagnosed GBM without any previous treatment (naïve Bev), and 14 were recurrent tumors after Bev administration (refractory Bev). The refractory Bev group included five specimens by autopsy in addition to nine recurrent tumors obtained from salvage surgery (Fig. 1A). As paired specimens from the same patient, 22 naïve Bev-refractory Bev specimens from 11 cases (including four autopsy specimens and seven salvage surgery specimens) and six effective Bev-refractory Bev specimens from three cases (including one autopsy specimen and two salvage surgery specimens) were obtained.
Fig. 1.
A Tissue sampling. Fifty-five tumor tissues obtained from three different settings: 15 tumor tissues were resected under neoadjuvant Bev (defined as “effective Bev”). Twenty-five tumor tissues were resected before Bev therapy (defined as “naïve Bev”). Fourteen tumor tissues were resected after Bev failure (defined as “refractory Bev”). Nine tumor tissues (seven from the naïve Bev group, and two from the effective Bev group) were obtained from salvage surgery. Five tumor tissues (four from the naïve Bev group, and one from the effective Bev group) were obtained from autopsy. B Treatment protocol of neoadjuvant Bev followed by surgery and postoperative adjuvant RT and TMZ combined with Bev. Surgery was performed in naïve, effective (just after preoperative neoadjuvant Bev), and refractory Bev periods. Bev bevacizumab, RT radiotherapy, TMZ temozolomide
Treatment protocol
All effective Bev patients were treated with preoperative neoadjuvant bevacizumab (neoBev) at 10 mg/kg on day 0. Surgical resection was performed 3–4 weeks after neoBev administration. Maintenance treatment with TMZ began 4 weeks after the completion of radiotherapy at a starting dose of 150 mg/m2 for 5 consecutive days of a 28-day cycle. All newly diagnosed GBM patients (naïve Bev) were treated with concomitant Stupp regimen and Bev after surgical resection (Supplement Table 1) [1], (Fig. 1B).
Study oversight
The protocol was approved by the ethics committee of Jikei University School of Medicine Kashiwa Hospital, Keio University School of Medicine, and Kagawa University Graduate School of Medicine. The study was approved by the Institutional Review Board (JKI18-052 for Jikei, N20160036 for Keio, 2018KH046 for Kagawa), and conducted in accordance with the Helsinki declaration. Written informed consent was obtained from all participants.
Immunohistochemistry
To assess changes in expression levels of angiogenic factors other than VEGF during Bev therapy, ANGPT1, ANGPT2, FGF2, EphA2, and PLGF were analyzed using 4-μm sections of formalin-fixed, paraffin-embedded tissues. Procedures were followed according to manufacturers’ protocols. Briefly, antigen retrieval was performed by a microwave method in 10 mM citrate buffer (pH; 6.0). After blocking with 2.5% normal horse serum (ImmPress Detection Systems; Vectorlabs, Burlingame, CA, USA) for 60 min, the sections were incubated overnight at 4 °C with anti-ANGPT1 (1:200, bs-0800R, BIOSS, MA, USA), anti-ANGPT2 (1:200, ab155106, abcam, Cambridge, UK), anti-FGF2 (1:100, sc-74412, Santa Cruz, TX, USA), anti-EphA2 (1:100, NBP2-02810, Novus Biologicals, CO, USA) or anti-PLGF (1:100, ab19666, abcam, Cambridge, UK) antibodies [22]. Immunoreactivity was visualized by the peroxidase–diaminobenzidine reaction. Expression levels of the five angiogenic factors were assessed by immunohistochemistry in tumor vessels consisting of vascular endothelial cells, pericytes, and other cells such as infiltrating macrophage surround the vessel walls, and five representative hot spots were selected under high-power observational fields (HPFs) of 400× magnification, 0.47 mm3. All experiments were assessed by five authors (TE, TT, RT, KO, and HS) and a consensus reached (Supplement Table 2).
MRI at recurrence
All patients were followed up regularly with MRI. The recurrent MRI pattern was classified into two settings, cT1-flare up and T2 diffuse/circumscribed [23, 24].
Statistical analyses
The Mann–Whitney U test was performed to statistically compare the levels of the angiogenic factors between any two groups. In paired samples, comparison was performed with a corresponding Wilcoxon signed ordered sum test. In addition, the Mann–Whitney U test was used to test association between the MRI pattern of recurrence and the expression of angiogenic factors.
Results
ANGPT1/ANGPT2
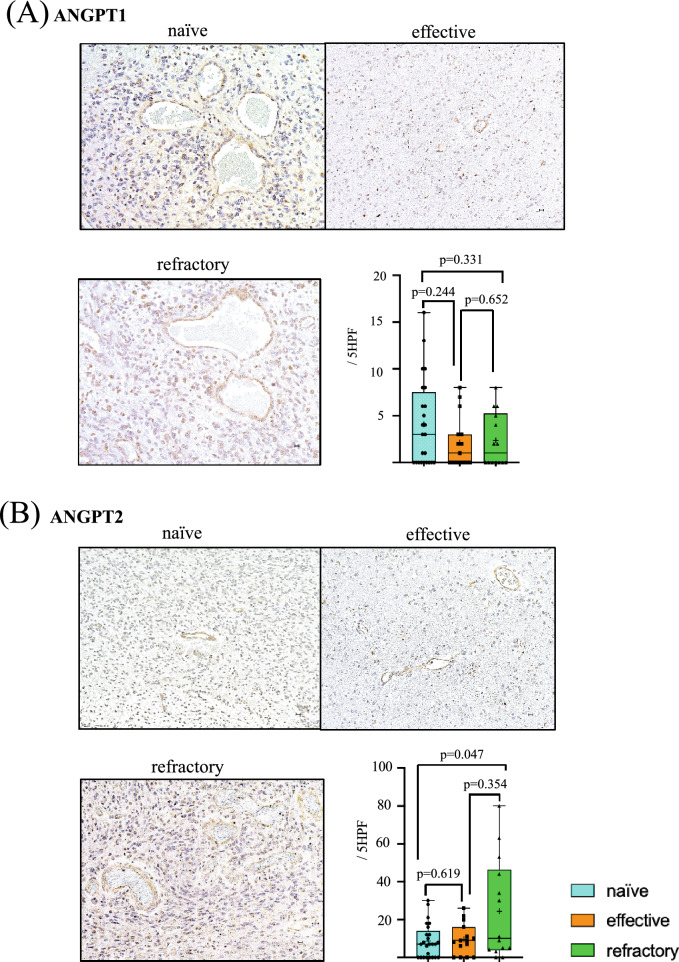
Both expression of ANGPT1 and ANGPT2 were intensely present in vascular endothelial cells especially in larger sized tumor vessels (≥ 15 µm) and some small vessels (< 15 µm). There was no statistical difference in ANGPT1 expression in vascular endothelial cells among groups (naïve vs. effective; p = 0.244, effective vs. refractory; p = 0.652, naïve vs. refractory; p = 0.331) (Fig. 2A).
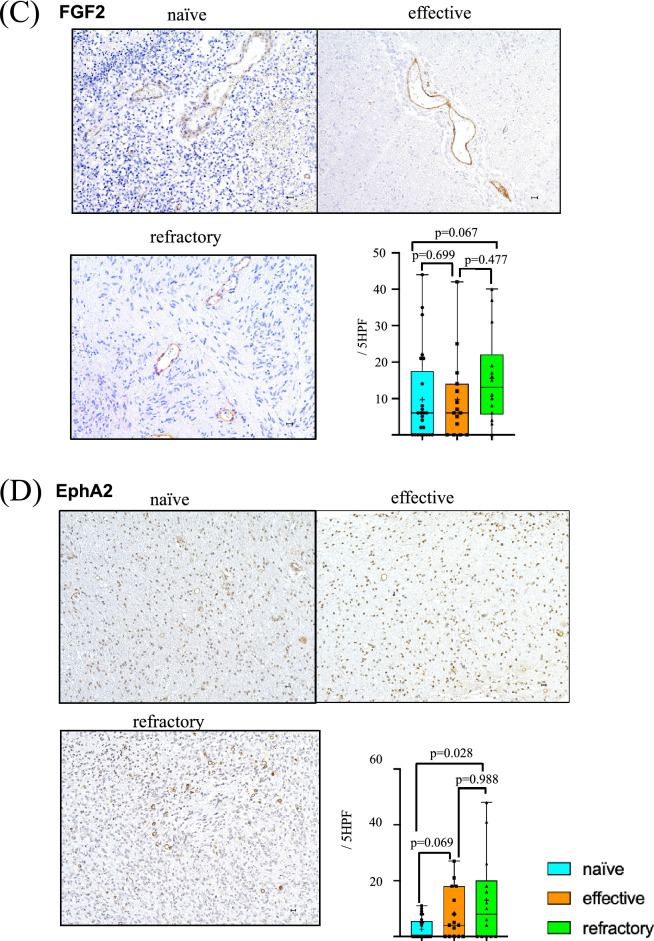
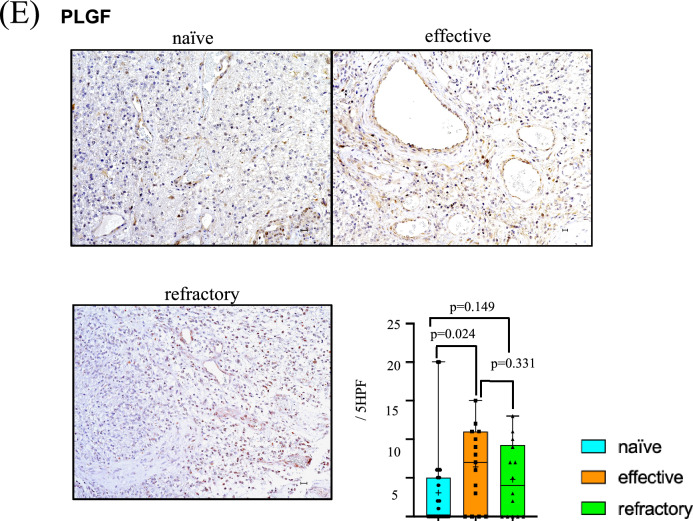
Fig. 2.
Levels of ANGPT1 (A), ANGPT2 (B), FGF2 (C), EphA2 (D) and PLGF (E) in tumor vessels. Immunohistochemistry photomicrographs shows naïve (upper left panel), effective (upper right panel) and refractory (bottom left panel). Numbers of positive vessels in tumor in five high power fields (5 HPF) were compared among naïve (blue), effective (orange), and refractory (green) Bev groups (bottom right panel)
However, there was significantly robust expression of ANGPT2 in tumor vessels in the refractory Bev group (24.36/5 HPF) compared with that in the naïve (8.68/5 HPF) and effective (9.60/5 HPF) Bev groups. There was also a significant difference between naïve Bev and refractory Bev groups (p = 0.047) (Fig. 2B).
FGF2
Compared with the other angiogenic factors, strong positive staining of FGF2 was detected in vascular endothelial cells especially in larger sized tumor vessels (≥ 15 µm). In contrast, there was very little staining in small tumor vessels (< 15 µm) (Fig. 2C). The expression level of FGF2 tended to increase from naïve (9.68/5 HPF) to effective (9.73/5 HPF) and refractory (15.50/5 HPF) Bev groups, although there was no significant difference.
EphA2
In contrast to FGF2, distribution of EphA2 expression was mostly localized in small vessels (< 15 µm). Expression of EphA2 was elevated in the refractory Bev group (12.93/5 HPF) compared with the naïve Bev (2.64/5 HPF) and the effective Bev (8.07/5 HPF) groups. There was a statistical difference between expression levels in the naïve Bev and refractory Bev groups (p = 0.028) (Fig. 2D).
PLGF
Distribution of PLGF expression was mostly localized in larger vessels (≥ 15 µm). Compared with tumor vessels in the naïve Bev group (3.08/5 HPF), expression level of PLGF was increased in vascular endothelial cells in effective (6.40/5 HPF) and refractory Bev (4.79/5 HPF) tumors. There was a statistical difference in the expression level of PLGF between effective Bev and naïve Bev groups (p = 0.024) (Fig. 2E).
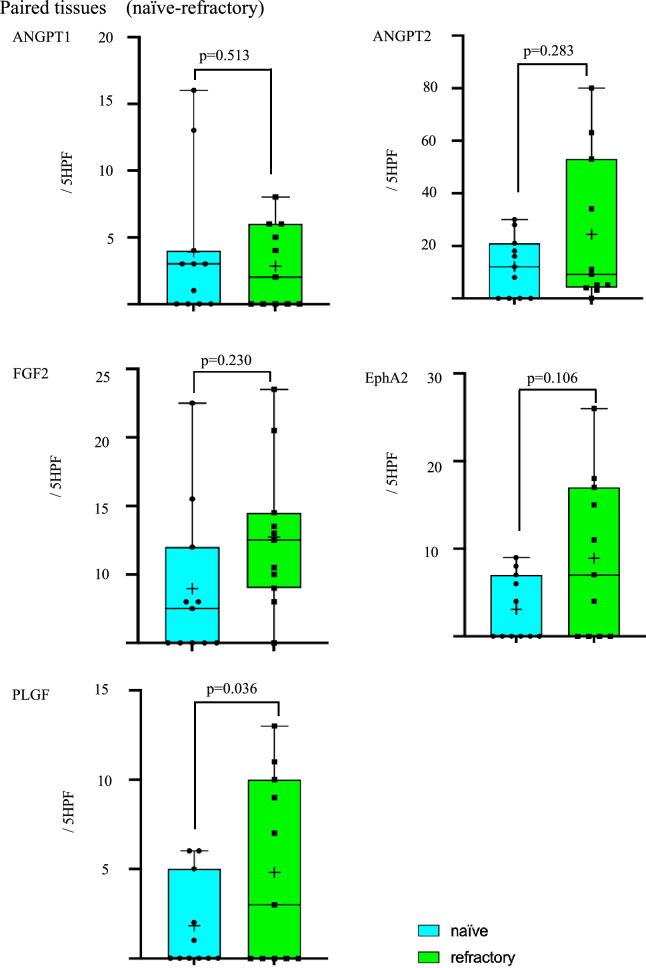
Comparative analyses using paired samples
Comparison of paired samples obtained from naïve and refractory Bev patients showed expression levels of PLGF to be higher in refractory Bev compared with naïve Bev samples (p = 0.036). In contrast, there were no significance differences in expression levels of ANGPT1, ANGPT2, FGF2 or EphA2 between naïve and refractory Bev groups (Fig. 3). In addition, three paired samples obtained from effective and refractory Bev, there were no significance differences in the expression levels of any angiogenic factors (Supplement Fig. 1).
Fig. 3.
Paired comparisons of ANGPT1, ANGPT2, FGF2, EFNA2, and PLGF by immunoreactivity in tumor vessels between naïve (blue) and refractory (green) Bev groups. Numbers of positive vessels were quantitated from five HPFs
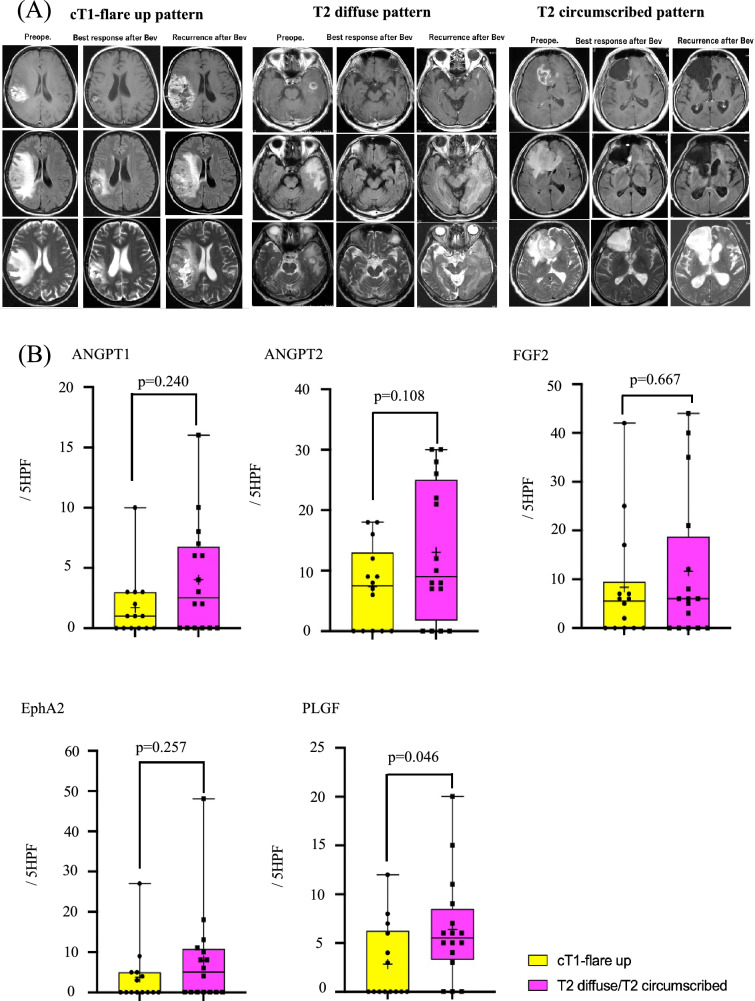
Expression of angiogenic factors according to recurrent pattern on MRI
Patterns of recurrence on MRI after Bev therapy were classified as previously described [5, 24] as cT1-flare up (n = 14) and T2 diffuse/circumscribed (n = 16) (Fig. 4A). In the refractory Bev group, expression levels of ANGPT1, ANGPT2, FGF2, EphA2 and PLGF in T2-diffuse/circumscribed tumors tended to be higher compared with levels in cT1-flare up tumors. In particular, there was statistically significant in the expression level of PLGF (p = 0.046) (Fig. 4B).
Fig. 4.
A Images of recurrent MRI (cT1-flare up and T2 diffuse/T2 circumscribed). Left panel shows cT1-flare up, middle panel shows T2 diffuse and right panel shows T2 circumscribed images. B Numbers of positive vessels of each recurrent pattern were quantitated in five HPFs. Yellow shows cT1-flare up and Pink shows T2-diffuse/T2 circumscribed pattern
Discussion
Preclinical studies of TMZ and Bev combination therapy for glioma demonstrated anti-tumor activity through inhibition of angiogenesis [25, 26]; however, clinical results were disappointing. Adaptation of the TME that leads to activation of redundant angiogenesis pathways is one mechanism that can lead to acquired resistance to anti-angiogenic therapies that target VEGF and its receptors [27]. Changes in angiogenic factors and cytokines have been described in patients treated with anti-angiogenic agents that target VEGF and tyrosine kinase [17, 28, 29]; however, few studies have analyzed paired samples from the same patients who underwent surgical resection during both naive and Bev-resistance stages [8, 9, 21]. In addition, it should be unique that we focused on expression of angiogenic factors other than VEGF as a salvage angiogenesis pathway in tumor vessel including vascular endothelial cell.
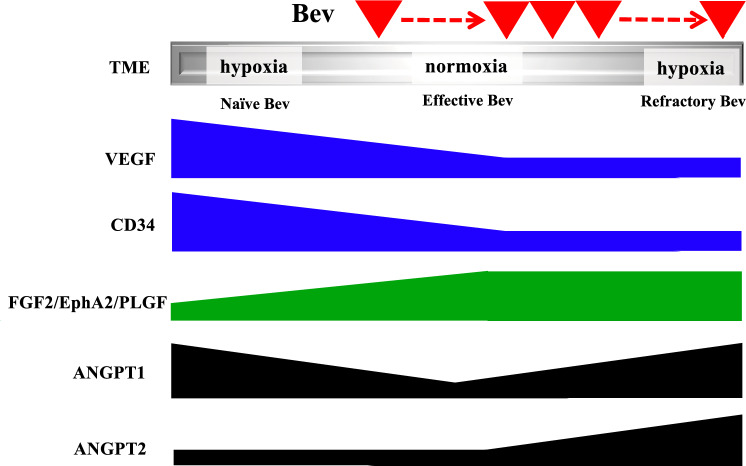
Clinical trials of VEGF-targeted therapy for GBM have shown upregulated serum levels of ANGPT2, EphA2, and FGF2 in the refractory period [17, 20, 28], while changes in the expression level of PLGF were controversial [8, 10, 21, 29]. Here, we report that expressions of ANGPT2, EphA2, and PLGF are upregulated in tumors resistant to Bev therapy, while expression levels of ANGPT1 and FGF2 evaluated by immunohistochemistry remain stable (Fig. 2A, B). We previously showed that the TME becomes normoxic in the effective Bev stage and hypoxic in the refractory Bev stage, regardless of VEGF suppression [5]. This raises the question of whether changes in tumor oxygenation during VEGF-targeting therapy affect alternative angiogenesis pathways. The present study demonstrates changes in expression levels of angiogenic factors in heterogeneous TME during Bev therapy (Fig. 5).
Fig. 5.
Scheme representing changes in VEGF, CD34, and alternative angiogenic factors in tumor vessels at naïve, effective and refractory Bev stages. FGF2, EphA2, and PLGF levels increase in the effective and refractory Bev stages. ANGPT1 tends to decrease in the effective Bev stage compared with the naïve Bev stage, and increase in the refractory Bev stage again, while ANGPT2 tends to increase in the refractory stage compared with naïve and effective Bev stages
FGF2, EphA2, and PLGF levels were upregulated in effective and refractory Bev stages under reduced vascular density (Fig. 2C, D, E) [5], regardless of therapeutic response. PLGF, belonging to the VEGF-family, was not altered between effective and refractory Bev stages [30]. PLGF was observed in tumor cells and vascular endothelial cells in hypoxic GBM, indicating that the TME under hypoxic conditions is also a source of PLGF [21]. PLGF elevation is significant during Bev therapy response in patients with metastatic colorectal cancer [31] and GBM [10], making it a reliable as a predictive biomarker in clinical outcomes.
Upregulation of EphA2 has potential as a novel immunotherapy for patients that are refractory to Bev [18–20, 32, 33]; however, the low expression level of EphA2 correlate with a favorable prognosis in the Cancer Genome Atlas GBM database [20], suggesting that EphA2 could also be a predictive biomarker and an alternative target for salvage therapy after Bev failure.
FGF2 is crucial for tumor angiogenesis and alternative angiogenic pathways during Bev therapy [7]. Multiple kinase inhibitors target VEGF, FGF, and PDGF receptor pathways to overcome resistance. Okamoto et al. investigated vascular structures and expression levels of angiogenic factors, including FGF2 and PDGF by paired comparison of initial surgery and autopsied samples after Bev failure [9]. Our present data demonstrated FGF2 to be upregulated at Bev therapy commencement, while a phase II study showed no significant change before or after Bev therapy, indicating no impact on clinical outcome [28, 34]. Further studies are needed to understand the significance of FGF2 induction in Bev therapy for GBM.
In naïve Bev, ANGPT1 facilitates vascular normalization and maturation during angiogenesis, whereas ANGPT2 has antagonist properties towards ANGPT1 [15, 35]. After VEGF blockade, expression level of ANGPT1 increased in a narrow therapeutic window during tumor oxygenation [13], while ANGPT2 induced vascular remodeling and sprouting under hypoxic TME at Bev resistance [14, 15, 36–38]. Our data show that ANGPT1 was downregulated in the effective Bev stage compared with the naïve Bev stage, while ANGPT2 remained unchanged until the refractory stage. Our previous and present data [5, 13, 16, 37, 38] indicate that ANGPT1 and ANGPT2 might be reciprocally regulated during tumor oxygenation but upregulated together in the refractory period (Fig. 5). It is also known that ANGPT2 compensates for VEGF inhibition by recruiting perivascular myeloid-derived suppressive cells and M2 macrophages [39, 40]. Upregulation of ANGPT2 is associated with T-cell exclusion, and blocking it promotes CD8+ T-cell infiltration, resulting in anti-tumor effects [41]. These evidences might support results of a clinical trial for bispecific antibodies targeting VEGF and ANGPT2 [42, 43], potentially supporting ANGPT2-targeted therapy combined with anti-VEGF therapy as a second-line therapy for patients with refractoriness of Bev. It also suggests that inhibition of ANGPT2 may overcome Bev resistance, and that combined immunotherapy may avoid tumor recurrence in hypoxic and immunosuppressive TME.
Radiographic comparison between enhancement and non-enhancement patterns in refractory Bev showed that there were no differences in expression levels of various angiogenic factors [23]. In the present study of recurrence patterns on MRI classified as T1-flare up and T2-diffuse pattern as previously described [5, 24], expression of all angiogenic factors examined in the present study was elevated in recurrent tumors with the T2-diffuse pattern on MRI. This discrepancy might be due to selection bias of autopsy samples as well as surgical resection of from non-enhancement pattern MRI tissue specimens in refractory Bev.
All angiogenic factors examined in the present study were upregulated in T2-diffuse/circumscribed pattern patients compared with cT1-flare up patients in pair cases of naïve-refractory, indicating that alternative angiogenesis pathways might be therapeutic targets when the non-enhancement pattern of recurrence on MRI occurs in patients with refractory to Bev.
In summary, this study reveals differential activation of salvage angiogenic pathways in surgical specimens, with FGF2, EphA2, and PLGF upregulated in tumor vessels, while ANGPT1 and ANGPT2 were downregulated and upregulated, respectively. Upregulation of angiogenic factors in hypoxic TME may compensate for a reduced blood supply, providing alternative therapeutic targets for recurrent GBM after VEGF-targeted therapy failure.
The study has several limitations. We assessed expression levels of angiogenic factors with focusing on tumor vessels including vascular endothelial cells, but it is difficult to detect expression levels of vascular endothelial cells accurately and strictly. It might be possible that pericytes and macrophage around tumor vessels were also included, consisting of tumor vessels. In addition, the present study was retrospective, limited to paired tissues from the same patients, and restricted to naïve and refractory Bev stages. The rarity of salvage surgery for recurrent GBM after Bev failure, RT, and TMZ makes achieving statistical significance difficult. In addition, paired samples were restricted to patients who underwent surgery for newly diagnosed GBM after preoperative neoadjuvant Bev [3]. Comparing paired samples between effective and refractory Bev stages is necessary to increase the study’s significance. Further studies using a larger number of patients are needed.
Conclusion
This study reports the analysis of alternative angiogenic factor pathways (non-VEGF pathways) at naive, effective, and refractory stages using paired specimens. There was a divergence in levels at the time of response between FGF2/EphA2/PLGF and ANGPT1/2. By immunohistochemical analyses, expression levels of angiogenic factors other than VEGF were more elevated in the MRI non-enhancement pattern compared with the enhancement pattern.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
TE, TT, and HS conceptualized, designed, and performed the study and wrote the manuscript. TT, JT, YY, and YA prepared the clinical samples. TT, RT, YY and JT assisted in the acquisition of data. JT, YY, YM, RI, YK, YA, MT, YM, and KM assisted with discussion and review of the manuscript. KO assisted in the pathological assessment. The first draft of the manuscript was written by TE, and all the authors approved the final version.
Funding
This work was supported by Keio University Academic Development Fund 2017 and 2018.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
This study was performed in line with the principles of the Declaration of Helsinki. Approval was granted by the Ethics Committee of Jikei University School of Medicine Kashiwa Hospital, Keio University School of Medicine, and Kagawa University Graduate School of Medicine. The study was approved by the Institutional Review Board (JKI18-052 for Jikei, N20160036 for Keio, 2018KH046 for Kagawa).
Consent to participate
Written informed consent was obtained from all the participants.
Consent to publish
The authors affirm that human research participants provided informed consent for publication of the images in Figs. 2 and 4.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
5/7/2024
A Correction to this paper has been published: 10.1007/s10014-024-00485-w
References
- 1.Chinot OL, Wick W, Mason W, et al. Bevacizumab plus radiotherapy-temozolomide for newly diagnosed glioblastoma. N Engl J Med. 2014;370:709–722. doi: 10.1056/NEJMoa1308345. [DOI] [PubMed] [Google Scholar]
- 2.Gilbert MR, Dignam JJ, Armstrong TS, et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N Engl J Med. 2014;370:699–708. doi: 10.1056/NEJMoa1308573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tamura R, Tanaka T, Miyake K, et al. Histopathological investigation of glioblastomas resected under bevacizumab treatment. Oncotarget. 2016;7:52423–52435. doi: 10.18632/oncotarget.9387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamura R, Tanaka T, Ohara K, et al. Persistent restoration to the immunosupportive tumor microenvironment in glioblastoma by bevacizumab. Cancer Sci. 2019;110(2):499–508. doi: 10.1111/cas.13889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamamoto Y, Tamura R, Tanaka T, et al. “Paradoxical” findings of tumor vascularity and oxygenation in recurrent glioblastomas refractory to bevacizumab. Oncotarget. 2017;8:103890–103899. doi: 10.18632/oncotarget.21978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Takei J, Fukasawa N, Tanaka T, et al. Impact of neoadjuvant bevacizumab on neuroradiographic response and histological findings related to tumor stemness and the hypoxic tumor microenvironment in glioblastoma: paired comparison between newly diagnosed and recurrent glioblastomas. Front Oncol. 2022;12:898614. doi: 10.3389/fonc.2022.898614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casanovas O, HicklinDJ, Bergers G et al (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8;299–309. [DOI] [PubMed]
- 8.Tabouret E, Denicolai E, Delfino C, et al. Changes in PlGF and MET-HGF expressions in paired initial and recurrent glioblastoma. J Neuro Oncol. 2016;130(3):431–437. doi: 10.1007/s11060-016-2251-5. [DOI] [PubMed] [Google Scholar]
- 9.Okamoto S, Nitta M, Murayama T, et al. Bevacizumab changes vascular structure and modulates the expression of angiogenic factors in recurrent malignant gliomas. Brain Tumor Pathol. 2016;33:129–136. doi: 10.1007/s10014-016-0248-6. [DOI] [PubMed] [Google Scholar]
- 10.Batcheler TT, Gerstner ER, Emblem KE, et al. Improved tumor oxygenation and survival in glioblastoma patients who show increased blood perfusion after cediranib and chemoradiation. Proc Natl Acad Sci USA. 2013;110(47):19059–19064. doi: 10.1073/pnas.1318022110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saharinen P, Eklund Lauri, Alitalo Kari (2017) Therapeutic targeting of the angiopoietin–TIE pathway. Nat Rev Drug Discov 16(9): 635–661. [DOI] [PubMed]
- 12.Sundberg C, Kowanetz M, Brown LW, et al. Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest. 2002;82(4):387–401. doi: 10.1038/labinvest.3780433. [DOI] [PubMed] [Google Scholar]
- 13.Winkler F, Kozin SV, Tong RT, et al. Kinetics of vascular normalization by VEGFR2 blockade governs brain tumor response to radiation: role of oxygenation, angiopoietin-1, and matrix metalloproteinases. Cancer Cell. 2004;6(6):553–563. doi: 10.1016/j.ccr.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Asahara T, Chen T, Takahashi T, et al. Tie2 receptor ligands, angiopoietin-1 and angiopoietin-2, modulate VEGF-induced postnatal neovascularization. Circ Res. 1998;83(3):233–240. doi: 10.1161/01.RES.83.3.233. [DOI] [PubMed] [Google Scholar]
- 15.Lauren J, Gunji Y, Alitalo K. Is angiopoietin-2 necessary for the initiation of tumor angiogenesis? Am J Pathol. 1998;153(5):1333–1339. doi: 10.1016/S0002-9440(10)65717-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maisonpierre PC, Suri C, Jones PF, et al. Angiopoietin-2, a natural antagonist for Tie2 that disrupts in vivo angiogenesis. Science. 1997;277(5322):55–60. doi: 10.1126/science.277.5322.55. [DOI] [PubMed] [Google Scholar]
- 17.Reardon DA, Lassman AB, Schiff D et al (2018) Phase 2 and biomarker study of Trebananib, an angiopoietin-blocking peptibody, with and without bevacizumab for patients with recurrent glioblastoma. Cancer 124(7):1438–1448 [DOI] [PubMed]
- 18.Affinito A, Quintavalle C, Esposito CL, et al. Targeting ephrin receptor tyrosine kinase A2 with a selective aptamer for glioblastoma stem cells. Mol Ther Nucleic Acid. 2020;20:176–185. doi: 10.1016/j.omtn.2020.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nakada M, Hayashi Y, Hamada J. Role of Eph/ephrin tyrosine kinase in malignant glioma. Neuro Oncol. 2011;13(11):1163–1170. doi: 10.1093/neuonc/nor102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Qazi MA, Vola P, Venugopal C, et al. Cotargeting ephrin receptor tyrosine kinases A2 and A3 in cancer stem cells reduces growth of recurrent glioblastoma. Cancer Res. 2018;78(17):5023–5037. doi: 10.1158/0008-5472.CAN-18-0267. [DOI] [PubMed] [Google Scholar]
- 21.Schneider K, Weyerbrock A, Doostkam S, et al. Lack of evidence for PlGF mediating the tumor resistance after anti-angiogenic therapy in malignant gliomas. J Neurooncol. 2015;121(2):269–278. doi: 10.1007/s11060-014-1647-3. [DOI] [PubMed] [Google Scholar]
- 22.Ezaki T, Sasaki H, Miwa T et al (2011) Molecular characteristics of pediatric non-ependymal, non‑pilocytic gliomas associated with resistance to temozolomide. Mol Med Rep 4(6):1101–1105. 10.3892/mmr.2011.573. [DOI] [PubMed]
- 23.DeLay M, Jahangiri A, Carbonell WS, et al. Microarray analysis verifies two distinct phenotypes of glioblastomas resistant to antiangiogenic therapy. Clin Cancer Res. 2012;18(10):2930–2942. doi: 10.1158/1078-0432.CCR-11-2390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nowosielski M, Wiestler B, Goebel G, et al. Progression types after antiangiogenic therapy are related to outcome in recurrent glioblastoma. Neurology. 2014;82(19):1684–1692. doi: 10.1212/WNL.0000000000000402. [DOI] [PubMed] [Google Scholar]
- 25.Mathieu V, Neve ND, Mercier ML, et al. Combining bevacizumab with temozolomide increases the antitumor efficacy of temozolomide in a human glioblastoma orthotopic xenograft model. Neoplasia. 2008;10:1381–1392. doi: 10.1593/neo.08928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grossman R, Brastianos H, Blakeley JO, et al. Combination of anti-VEGF therapy and temozolomide in two-experimental human glioma models. J Neuro Oncol. 2014;116:59–65. doi: 10.1007/s11060-013-1268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Montemagno C, Pages G (2020) Resistance to anti-angiogenic therapies: a mechanism depending on the time of exposure to the drugs. Front Cell Dev Biol. 10.3389/fcell.2020.00584.eCollection. [DOI] [PMC free article] [PubMed]
- 28.Lee EQ, Muzikansky A, Duda DG, et al. Phase II trial of ponatinib in patients with bevacizumab-refractory globlastoma. Cancer Med. 2019;8:5988–5994. doi: 10.1002/cam4.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee J, Park DY, Park DY, et al. Angiopoietin-1 suppresses choroidal neovascularization and vascular leakage. Invest Ophthalmol Vis Sci. 2014;55(4):2191–2199. doi: 10.1167/iovs.14-13897. [DOI] [PubMed] [Google Scholar]
- 30.Sawano A, Takahashi T, Tamaguchi S, et al. Flt-1 but not KDR/Flk-1 tyrosine kinase is a receptor for placenta growth factor, which is related to vascular endothelial growth factor. Cell Growth Differ. 1996;7(2):213–221. [PubMed] [Google Scholar]
- 31.Cubillo A, Galleago RA, Munoz M, et al. Dynamic angiogenic switch as predictor of response to chemotherapy-bevacizumab in patients with metastatic colorectal cancer. Am J Clin Oncol. 2019;42:56–59. doi: 10.1097/COC.0000000000000474. [DOI] [PubMed] [Google Scholar]
- 32.Wykosky J, Gibo DM, Stanton C, et al. Eph A2 as a novel molecular marker and target in glioblastoma multiforme. Mol Cancer Res. 2005;3(10):541–551. doi: 10.1158/1541-7786.MCR-05-0056. [DOI] [PubMed] [Google Scholar]
- 33.Yi Z, Prinzing B, Cao F, et al. Optimizing EphA2-CAR T cells for the adoptive immunotherapy for glioma. Mol Ther Methods Clin Dev. 2018;9:70–78. doi: 10.1016/j.omtm.2018.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yao JC, Phan A, Hoff PM, et al. Targeting vascular endothelial growth factor in advanced carcinoid tumor: a random assignment phase II study of depot octreotide with bevacizumab and pegylated interferon alpha-2b. J Clin Oncol. 2008;26(8):1316–1323. doi: 10.1200/JCO.2007.13.6374. [DOI] [PubMed] [Google Scholar]
- 35.Stratmann A, et al. Cell type-specific expression of angiopoietin-1 and angiopoietin-2 suggests a role in glioblastoma angiogenesis. Am J Pathol. 1998;153(5):1459–1466. doi: 10.1016/S0002-9440(10)65733-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Crawford Y, Risau W, Plate KH. Tumor and stromal pathways mediating refractoriness/resistance to anti-angiogenic therapies. Trends Pharmacol Sci. 2009;30:624–630. doi: 10.1016/j.tips.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Ding H, Roncari L, Wu X, et al. Expression and hypoxic regulation of angiopoietins in human astrocytes. Neuro-Oncol. 2001;3:1–10. doi: 10.1093/neuonc/3.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lund EL, Hog A, Olsen MWB, et al. Differential regulation of VEGF, HIF-1αand angiopoietin-1, -2, and -4 by hypoxia and ionizing radiation in human glioblastoma. Int J Cancer. 2004;108:833–838. doi: 10.1002/ijc.11662. [DOI] [PubMed] [Google Scholar]
- 39.Scholz A, Harter PH, Cremer S et al (2016) Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma EMBO Mol Med 8(1):39–57. 10.15252/emmm.201505505. [DOI] [PMC free article] [PubMed]
- 40.Baker HE, Paget JTE, Khan AA, et al. The tumour microenvironment after radiotherapy: mechanisms of resistance and recurrence. Nat Rev Cancer. 2015;15(7):409–425. doi: 10.1038/nrc3958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Park HM, Shiva A, Cummings P, et al. Angiopoietin-2-dependent spatial vascular destabilization promotes T-cell exclusion and limits immunotherapy in melanoma. Cancer Res. 2023;83:1968–1983. doi: 10.1158/0008-5472.CAN-22-2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Doppalaudi VR, Dingguo JH, Liu D, et al. Chemical generation of bispecific antibodies. Proc Natl Acad Sci USA. 2010;107(52):22611–22616. doi: 10.1073/pnas.1016478108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kloepper J, Riedemann L, Amoozgar Z, et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc Natl Acad Sci USA. 2016;113(16):4476–4481. doi: 10.1073/pnas.1525360113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.