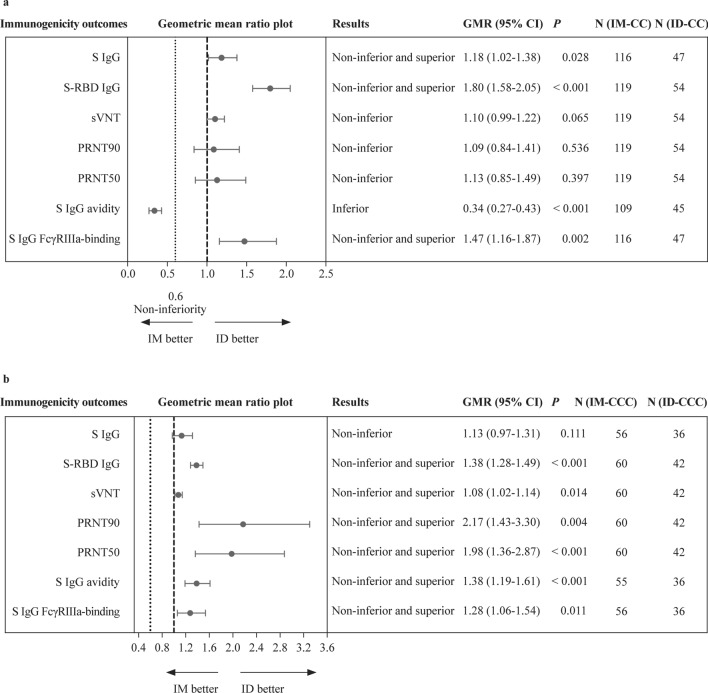
Fig. 1.
Superiority and non-inferiority hypothesis testing of humoral immunogenicity against wild type SARS-CoV-2 post-dose 2 and post-dose 3 of vaccination in the evaluable analysis population. Adolescents receiving 2 doses of CoronaVac administered intramuscularly (IM-CC) or intradermally (ID-CC) (a) and 3 doses of CoronaVac administered intramuscularly (IM-CCC) or intradermally (ID-CCC) (b) were tested for humoral immunogenicity outcomes. Dots and error bars show GMR estimates and two-sided 95% CI, respectively. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, GMR geometric mean ratio, ID intradermal, IM intramuscular, N nucleocapsid protein, S spike protein, RBD receptor-binding domain, sVNT surrogate virus neutralisation test, PRNT plaque reduction neutralisation titre, FcγRIIIa Fcγ receptor IIIa, IgG immunoglobulin G, CI confidence interval