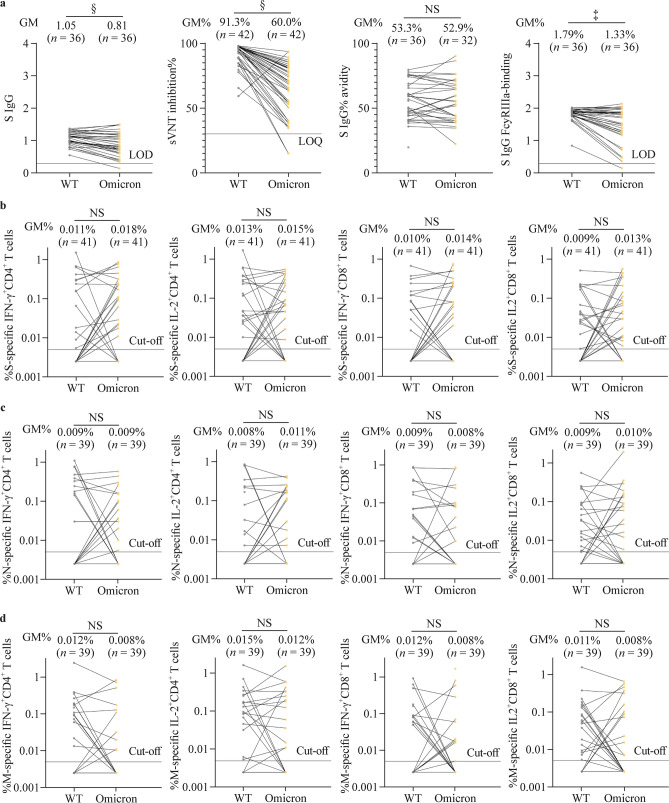
Fig. 4.
Omicron variant-specific humoral and cellular immunogenicity post-dose 3 of CoronaVac administered intradermally in the evaluable analysis population. Humoral (a) and (b–d) cellular immunogenicity outcomes against WT SARS-CoV-2 and the Omicron variant post-dose 3 of CoronaVac administered intradermally. Data labels and centre lines show GM estimates, and error bars show 95% CI. P values were derived from two-tailed unpaired t test after natural logarithmic transformation. SARS-CoV-2 severe acute respiratory syndrome coronavirus 2, GM geometric mean, WT wild type, S spike protein, N nucleocapsid protein, M membrane protein, IgG immunoglobulin G, sVNT surrogate virus neutralisation test, FcγRIIIa Fcγ receptor IIIa, LOD limit of detection, IFN-γ interferon-γ, IL-2 interleukin-2, CI confidence interval, NS no significant difference. ‡P < 0.001; §P < 0.0001